Forced dimerization of gp130 leads to constitutive STAT3 activation, cytokine-independent growth, and blockade of differentiation of embryonic stem cells
- PMID: 16624864
- PMCID: PMC1483035
- DOI: 10.1091/mbc.e05-12-1129
Forced dimerization of gp130 leads to constitutive STAT3 activation, cytokine-independent growth, and blockade of differentiation of embryonic stem cells
Abstract
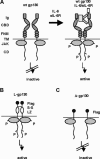
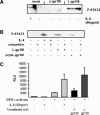
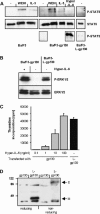
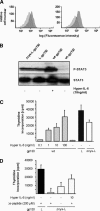
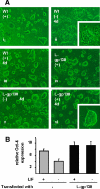
The mode of activation of glycoprotein 130 kDa (gp130) and the transmission of the activation status through the plasma membrane are incompletely understood. In particular, the molecular function of the three juxtamembrane fibronectin III-like domains of gp130 in signal transmission remains unclear. To ask whether forced dimerization of gp130 is sufficient for receptor activation, we replaced the entire extracellular portion of gp130 with the c-jun leucine zipper region in the chimeric receptor protein L-gp130. On expression in cells, L-gp130 stimulates ligand-independent signal transducer and activator of transcription (STAT) 3 and extracellular signal-regulated kinase 1/2 phosphorylation. gp130 activation could be abrogated by the addition of a competing peptide comprising the leucine zipper region of c-fos. When stably expressed in the interleukin-3-dependent Ba/F3 murine pre-B-cells, these cells showed constitutive STAT3 activation and cytokine-independent growth over several months. Because gp130 stimulation completely suppressed differentiation of murine embryonic stem cells in vitro, we also stably expressed L-gp130 in these cells, which completely blocked their differentiation in the absence of cytokine stimulation and was consistent with high constitutive expression levels of the stem cell factor OCT-4. Thus, L-gp130 can be used in vitro and in vivo to mimic constitutive and ligand-independent activation of gp130 and STAT3, the latter of which is frequently observed in neoplastic diseases.
Figures

References
-
- Auguste P., Guillet C., Fourcin M., Olivier C., Veziers J., Pouplard Barthelaix A., Gascan H. Signaling of type II oncostatin M receptor. J. Biol. Chem. 1997;272:15760–15764. - PubMed
-
- Behncken S. N., Billestrup N., Brown R., Amstrup J., Conway-Campbell B., Waters M. J. Growth hormone (GH)-independent dimerization of GH receptor by a leucine zipper results in constitutive activation. J. Biol. Chem. 2000;275:17000–17007. - PubMed
-
- Boulanger M. J., Chow D. C., Brevnova E. E., Garcia K. C. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300:2101–2104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

