Amplified fragment length polymorphism mapping of quantitative trait loci for malaria parasite susceptibility in the yellow fever mosquito Aedes aegypti
- PMID: 16624910
- PMCID: PMC1526673
- DOI: 10.1534/genetics.105.055178
Amplified fragment length polymorphism mapping of quantitative trait loci for malaria parasite susceptibility in the yellow fever mosquito Aedes aegypti
Abstract
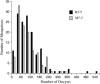
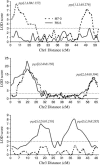
The yellow fever mosquito Aedes aegypti has been the subject of extensive genetic research due to its medical importance and the ease with which it can be manipulated in the laboratory. A molecular genetic linkage map was constructed using 148 amplified fragment length polymorphism (AFLP) and six single-strand conformation polymorphism (SSCP) markers. Eighteen AFLP primer combinations were used to genotype two reciprocal F2 segregating populations. Each primer combination generated an average of 8.2 AFLP markers eligible for linkage mapping. The length of the integrated map was 180.9 cM, giving an average marker resolution of 1.2 cM. Composite interval mapping revealed a total of six QTL significantly affecting Plasmodium susceptibility in the two reciprocal crosses of Ae. aegypti. Two common QTL on linkage group 2 were identified in both crosses that had similar effects on the phenotype, and four QTL were unique to each cross. In one cross, the four main QTL accounted for 64% of the total phenotypic variance, and digenic epistasis explained 11.8% of the variance. In the second cross, the four main QTL explained 66% of the variance, and digenic epistasis accounted for 16% of the variance. The actions of these QTL were either dominance or underdominance. Our results indicated that at least three new QTL were mapped on chromosomes 1 and 3. The polygenic nature of susceptibility to P. gallinaceum and epistasis are important factors for significant variation within or among mosquito strains. The new map provides additional information useful for further genetic investigation, such as identification of new genes and positional cloning.
Figures

References
-
- Abraham, E. G., and M. Jacobs-Lorena, 2004. Mosquito midgut barriers to malaria parasite development. Insect Biochem. Mol. Biol. 34: 667–671. - PubMed
-
- Alavi, Y., M. Arai, J. Mendoza, M. Tufet-Bayona, R. Sinha et al., 2003. The dynamics of interactions between Plasmodium and the mosquito: a study of the infectivity of Plasmodium berghei and Plasmodium gallinaceum, and their transmission by Anopheles stephensi, Anopheles gambiae and Aedes aegypti. Int. J. Parasitol. 33: 933–943. - PubMed
-
- Antolin, M. F., C. F. Bosio, J. Cotton, W. Sweeney, M. R. Strand et al., 1996. Intensive linkage mapping in a wasp (Bracon hebetor) and a mosquito (Aedes aegypti) with single-strand conformation polymorphism analysis of random amplified polymorphic DNA markers. Genetics 143: 1727–1738. - PMC - PubMed
-
- Bassam, B. J., G. Caetano-Anolles and P. M. Gresshoff, 1991. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal. Biochem. 196: 80–83. - PubMed
-
- Beernsten, B. T., D. W. Severson, J. A. Klinkhammer, V. A. Kassner and B. M. Christensen, 1995. Aedes aegypti: a quantitative trait locus (QTL) influencing filarial worm intensity is linked to QTL for susceptibility to other mosquito-borne pathogens. Exp. Parasitol. 81: 355–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

