Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex
- PMID: 16624936
- PMCID: PMC3070309
- DOI: 10.1523/JNEUROSCI.0175-06.2006
Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex
Abstract
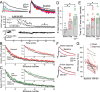
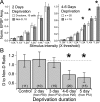
Whisker deprivation weakens excitatory layer 4 (L4) inputs to L2/3 pyramidal cells in rat primary somatosensory (S1) cortex, which is likely to contribute to whisker map plasticity. This weakening has been proposed to represent long-term depression (LTD) induced by sensory deprivation in vivo. Here, we studied the synaptic expression mechanisms for deprivation-induced weakening of L4-L2/3 inputs and assessed its similarity to LTD, which is known to be expressed presynaptically at L4-L2/3 synapses. Whisker deprivation increased the paired pulse ratio at L4-L2/3 synapses and slowed the use-dependent block of NMDA receptor currents by MK-801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate], indicating that deprivation reduced transmitter release probability at these synapses. In contrast, deprivation did not alter either miniature EPSC amplitude in L2/3 neurons or the amplitude of quantal L4-L2/3 synaptic responses measured in strontium, indicating that postsynaptic responsiveness was unchanged. In young postnatal day 12 (P12) rats, at least 4 d of deprivation were required to significantly weaken L4-L2/3 synapses. Similar weakening occurred when deprivation began at older ages (P20), when synapses are mostly mature, indicating that weakening is unlikely to represent a failure of synaptic maturation but instead represents a reduction in the strength of existing synapses. Thus, whisker deprivation weakens L4-L2/3 synapses by decreasing presynaptic function, similar to known LTD mechanisms at this synapse.
Figures





References
-
- Allen CB, Celikel T, Feldman DE (2003). Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci 6:291–299. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A (1992). Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol 68:1345–1358. - PubMed
-
- Bear MF, Cooper LN, Ebner FF (1987). A physiological basis for a theory of synapse modification. Science 237:42–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
