Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex
- PMID: 16624937
- PMCID: PMC3071735
- DOI: 10.1523/JNEUROSCI.0176-06.2006
Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex
Abstract
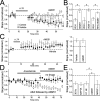
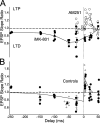
Many cortical synapses exhibit spike timing-dependent plasticity (STDP) in which the precise timing of presynaptic and postsynaptic spikes induces synaptic strengthening [long-term potentiation (LTP)] or weakening [long-term depression (LTD)]. Standard models posit a single, postsynaptic, NMDA receptor-based coincidence detector for LTP and LTD components of STDP. We show instead that STDP at layer 4 to layer 2/3 synapses in somatosensory (S1) cortex involves separate calcium sources and coincidence detection mechanisms for LTP and LTD. LTP showed classical NMDA receptor dependence. LTD was independent of postsynaptic NMDA receptors and instead required group I metabotropic glutamate receptors and calcium from voltage-sensitive channels and IP3 receptor-gated stores. Downstream of postsynaptic calcium, LTD required retrograde endocannabinoid signaling, leading to presynaptic LTD expression, and also required activation of apparently presynaptic NMDA receptors. These LTP and LTD mechanisms detected firing coincidence on approximately 25 and approximately 125 ms time scales, respectively, and combined to implement the overall STDP rule. These findings indicate that STDP is not a unitary process and suggest that endocannabinoid-dependent LTD may be relevant to cortical map plasticity.
Figures







References
-
- Abbott LF, Nelson SB (2000). Synaptic plasticity: taming the beast. Nat Neurosci 3:Suppl, 1178–1183. - PubMed
-
- Allen CB, Celikel T, Feldman DE (2003). Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci 6:291–299. - PubMed
-
- Anwyl R (1999). Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29:83–120. - PubMed
-
- Artola A, Singer W (1993). Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci 16:480–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
