Peripheral antinociceptive effects of exogenous and immune cell-derived endomorphins in prolonged inflammatory pain
- PMID: 16624955
- PMCID: PMC6673991
- DOI: 10.1523/JNEUROSCI.4349-05.2006
Peripheral antinociceptive effects of exogenous and immune cell-derived endomorphins in prolonged inflammatory pain
Abstract
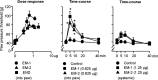
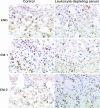
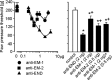
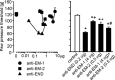
Endomorphins (EMs) are endogenous selective mu-opioid receptor agonists. Their role in inflammatory pain has not been fully elucidated. Here we examine peripheral antinociception elicited by exogenously applied EM-1 and EM-2 and the contribution of EM-containing leukocytes to stress- and corticotropin-releasing factor (CRF)-induced antinociception. To this end, we applied behavioral (paw pressure) testing, radioligand binding, immunohistochemistry, and flow cytometry in rats with unilateral hindpaw inflammation induced with Freund's adjuvant. EMs injected directly into both hindpaws produced antinociception exclusively in inflamed paws. This was blocked by locally applied mu-receptor-selective (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2) but not kappa-receptor-selective (nor-binaltorphimine) antagonists. Delta-receptor antagonists (naltrindole and N,N-diallyl-Tyr-Aib-Aib-Phe-Leu) did not influence EM-1-induced but dose-dependently decreased EM-2-induced antinociception. Antibodies against beta-endorphin, methionine-enkephalin, or leucine-enkephalin did not significantly change EM-2-induced antinociception. Both EMs displaced binding of [3H]-[D-Ala2,N-Me-Phe4,Gly5-ol]enkephalin to mu-receptors in dorsal root ganglia (DRG). Using [3H]-naltrindole or [(125)I]-[D-Pen2,5]-enkephalin, no detectable delta-binding was found in DRG of inflamed hindlimbs. Numerous beta-endorphin-containing and fewer EM-1- and EM-2-containing leukocytes were detected in subcutaneous tissue of inflamed paws. Leukocyte-depleting serum decreased the number of immigrating opioid-containing immune cells and attenuated swim stress- and CRF-induced antinociception in inflamed paws. Both forms of antinociception were strongly attenuated by anti-beta-endorphin and to a lesser degree by anti-EM-1 and anti-EM-2 antibodies injected into inflamed paws. Together, exogenously applied and immune cell-derived EMs alleviate prolonged inflammatory pain through selective activation of peripheral opioid receptors. Exogenous EM-2 in addition to mu-receptors also activates peripheral delta-receptors, which does not involve actions via other opioid peptides.
Figures

References
-
- Antunes Bras J, Becker C, Bourgoin S, Lombard M, Cesselin F, Hamon M, Pohl M (2001). Met-enkephalin is preferentially transported into the peripheral processes of primary afferent fibres in both control and HSV1-driven proenkephalin A overexpressing rats. Neuroscience 103:1073–1083. - PubMed
-
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, Xiao HS, Xu ZQ, He C, Hökfelt T, Zhou Z, Zhang X (2003). Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron 37:121–133. - PubMed
-
- Brack A, Rittner HL, Machelska H, Shaqura M, Mousa SA, Labuz D, Zollner C, Schafer M, Stein C (2004a). Endogenous peripheral antinociception in early inflammation is not limited by the number of opioid-containing leukocytes but by opioid receptor expression. Pain 108:67–75. - PubMed
-
- Brack A, Rittner HL, Machelska H, Leder K, Mousa SA, Schafer M, Stein C (2004b). Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain 112:229–238. - PubMed
-
- Buzas B, Cox BM (1997). Quantitative analysis of mu and delta opioid receptor gene expression in rat brain and peripheral ganglia using competitive polymerase chain reaction. Neuroscience 76:479–489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
