Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex
- PMID: 16624959
- PMCID: PMC6674016
- DOI: 10.1523/JNEUROSCI.0252-06.2006
Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex
Abstract
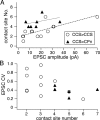
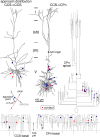
Corticostriatal pyramidal cells are heterogeneous in the frontal cortex. Here, we show that subpopulations of corticostriatal neurons in the rat frontal cortex are selectively connected with each other based on their subcortical targets. Using paired recordings of retrogradely labeled cells, we investigated the synaptic connectivity between two projection cell types: those projecting to the pons [corticopontine (CPn) cell], often with collaterals to the striatum, and those projecting to both sides of the striatum but not to the pons [crossed corticostriatal (CCS) cell]. The two types were morphologically differentiated in regard to their apical tufts. The dendritic morphologies of CCS cells were correlated with their somatic depth within the cortex. CCS cells had reciprocal synaptic connections with each other and also provided synaptic input to CPn cells. However, connections from CPn to CCS cells were rarely found, even in pairs showing CCS to CPn connectivity. Additionally, CCS cells preferentially innervated the basal dendrites of other CCS cells but made contacts onto both the basal and apical dendrites of CPn cells. The amplitude of synaptic responses was to some extent correlated with the contact site number. Ratios of the EPSC amplitude to the contact number tended to be larger in the CCS to CCS connection. Therefore, our data demonstrate that these two types of corticostriatal cells distinct in their dendritic morphologies show directional and domain-dependent preferences in their synaptic connectivity.
Figures








References
-
- Albin RL, Young AB, Penney JB (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375. - PubMed
-
- Alexander GE, Crutcher MD (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271. - PubMed
-
- Anderson J, Lampl I, Reichova I, Carandini M, Ferster D (2000). Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nat Neurosci 3:617–621. - PubMed
-
- Bauswein E, Fromm C, Preuss A (1989). Corticostriatal cells in comparison with pyramidal tract neurons: contrasting properties in the behaving monkey. Brain Res 493:198–203. - PubMed
-
- Christophe E, Doerflinger N, Lavery DJ, Molnar Z, Charpak S, Audinat E (2005). Two populations of layer V pyramidal cells of the mouse neocortex: development and sensitivity to anesthetics. J Neurophysiol 94:3357–3367. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
