Ipratropium bromide versus short acting beta-2 agonists for stable chronic obstructive pulmonary disease
- PMID: 16625543
- PMCID: PMC6513456
- DOI: 10.1002/14651858.CD001387.pub2
Ipratropium bromide versus short acting beta-2 agonists for stable chronic obstructive pulmonary disease
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a condition associated with high morbidity, mortality and cost to the community. Patients often report symptomatic improvement with short-acting beta-2 agonists (SABA) and anticholinergic bronchodilator medications, and both are recommended in COPD guidelines. These medications have different mechanisms of action and therefore could have an additive effect when combined.
Objectives: To compare the relative efficacy and safety of regular long term use (at least four weeks) of ipratropium bromide and short- acting beta-2 agonist therapy in patients with stable COPD.
Search strategy: The Cochrane Airways Group Specialised Register of Trials was searched. Bibliographies were checked to identify relevant cross-references. Drug companies were contacted for relevant trial data. The searches are current to August 2005.
Selection criteria: All randomised controlled trials comparing at least 4 weeks of treatment with an anticholinergic agent (ipratropium bromide) alone or in combination with a beta-2 agonist (short acting) versus the beta-2 agonist alone, delivered via metered dose inhaler or nebuliser, in non-asthmatic adult subjects with stable COPD.
Data collection and analysis: Data extraction and study quality assessment was performed independently by three reviewers. Authors of studies and relevant manufacturers were contacted if data were missing.
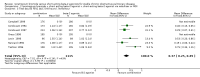
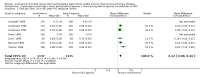
Main results: Eleven studies (3912 participants) met the inclusion criteria of the review. Small benefits of ipratropium over a short-acting beta-2 agonist were demonstrated on lung function outcomes. There were small benefits in favour of ipratropium on quality of life (HRQL), as well as a reduction in the requirement for oral steroids. Combination therapy with ipratropium plus a short-acting beta-2 agonist conferred benefits over a short-acting beta-2 agonist alone in terms of post-bronchodilator lung function. There was no significant benefit of combination therapy in subjective improvements in HRQL, but again there was a reduction in the requirement for oral steroids.
Authors' conclusions: The available data from the trials included in this review suggest that the advantage of regular long term use of ipratropium alone or in combination with a short-acting beta-2 agonist or over a beta-2 agonist alone are small, if the aim is to improve lung function, symptoms and exercise tolerance. Until further data are available, the strategy of providing a short-acting beta-2 agonist on a PRN basis, and then either continuing with the short-acting beta-2 agonist regularly or conducting an "n of 1" trial of regular beta-2 agonist or regular anticholinergic to determine the treatment that gives the best relief of symptoms (and continuing with it), would seem cost effective. This strategy does need formal evaluation. Patient preference is also important, as is the relative importance of avoiding the use of systemic corticosteroids.
Conflict of interest statement
None known.
Figures



















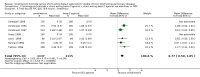






Update of
References
References to studies included in this review
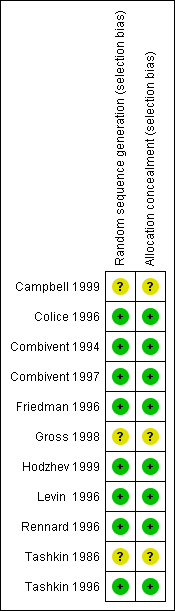
Campbell 1999 {published data only}
-
- Campbell S. For COPD a combination of ipratropium bromide and albuterol sulfate is more effective than albuterol base. Archives Internal Medicine 1999;159(Jan 25):156‐60. - PubMed
Colice 1996 {published and unpublished data}
-
- Brown D, et al. A randomized, double blind, parallel, multi‐centre comparison of inhalation solution with albuterol inhalation solution following single‐dose and chronic administration (85 days) in patients with chronic obstructive pulmonary disease. Boehringer Ingelheim unpublished report USA U91‐0865 1991.
-
- Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. The American Journal of Medicine 1996;100(1A):11S‐18S. - PubMed
-
- Rennard SI, Serby CW, Ghafouri M, Johnson PA, Friedman M. Extended therapy with ipratropium is associated with improved lung function in patients with COPD. A retrospective analysis of data from seven clinical trials. Chest 1996;110:62‐70. - PubMed
Combivent 1994 {published and unpublished data}
-
- "COMBIVENT" Inhalation Aerosol Study Group. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. Chest 1994;105(5):1411‐9. - PubMed
-
- Alexander KM, et al. A randomized, double blind, parallel, multi‐centre comparison of Combivent (ipratropium bromide and albuterol sulfate) inhalation aerosol with its components following single‐dose and chronic administration (85 days) in patients with chronic obstructive pulmonary disease. Boehringer Ingelheim unpublished report: USA U92‐0462. 1992.
Combivent 1997 {published data only}
-
- Alexander KM, et al. A randomized, double blind, parallel, multi‐centre comparison of Combivent (ipratropium bromide and albuterol sulfate) inhalation solution with its components following single‐dose and chronic administration (85 days) in patients with chronic obstructive pulmonary disease. Boehringer Ingelheim unpublished report: USA U92‐0801 1992.
-
- Rennard SI, Serby CW, Ghafouri M, Johnson PA, Friedman M. Extended therapy with ipratropium is associated with improved lung function in patients with COPD. A retrospective analysis of data from seven clinical trials. Chest 1996;110:62‐70. - PubMed
-
- The COMBIVENT Inhalation Study Group. Routine nebulized ipratropium and albuterol together are better than either alone in COPD. Chest 1997;112(6):1514‐21. - PubMed
Friedman 1996 {published and unpublished data}
-
- Brown D, et al. A randomized, double blind, parallel, multi‐centre comparison of Atrovent (ipratropium bromide) inhalation solution with metaproterenol inhalation solution following single‐dose and chronic administration (85 days) in patients with chronic obstructive pulmonary disease. Boehringer Ingelheim unpublished report USA U91‐0866. 1991.
-
- Friedman M. A multicenter study of nebulized bronchodilator solutions in chronic obstructive pulmonary disease. The American Journal of Medicine 1996;100(1A):30s‐39s. - PubMed
Gross 1998 {published data only}
-
- Gross N, Tashkin D, Miller R, Oren J, Coleman W, Linberg S, et al. Inhalation by nebulization of albuterol‐ipratropium combination (Dey Combination) is superior to either agent alone in the treatment of chronic obstructive pulmonary disease. Respiration 1998;65:354‐62. - PubMed
Hodzhev 1999 {published data only}
-
- Hodzhev B, Kostianev S, Todorov I, Belev G, Kartev S. Individual results of treatment of COPD with low doses of fenoterol compared with treatment with ipratropium bromide. Folia Med (Plovdiv) 1999;41:46‐52. - PubMed
Levin 1996 {published and unpublished data}
-
- Levin DC, Little KS, Laughlin KR, Galbraith JM, Gustman PM, et al. Addition of anticholinergic solution prolongs bronchodilator effect of Beta2 agonists in patients with chronic obstructive pulmonary disease. The American Journal of Medicine 1996;100(1A):40s‐48s. - PubMed
Rennard 1996 {published and unpublished data}
-
- Rennard SI, Serby CW, Ghafouri M, Johnson PA, Friedman M. Extended therapy with ipratropium is associated with improved lung function in patients with COPD. A retrospective analysis of data from seven clinical trials. Chest 1996;110:62‐70. - PubMed
Tashkin 1986 {published data only}
-
- Tashkin DP, Ashutosh K, Bleeker ER, Britt EJ, Cugell DW, Cumminsky JM, et al. Comparison of the anticholinergic bronchodilator ipratropium bromide with metaproterenol in chronic obstructive pulmonary disease. A 90‐Day Multi‐Centre Study. The American Journal of Medicine 1986;81(Suppl 5A):81‐90. - PubMed
Tashkin 1996 {published and unpublished data}
-
- Tashkin DP, Bleeker E, Braun S, Campbell S, DeGraff AC, Hudgell DW, et al. Results of a multicentre study of nebulised inhalant bronchodilator solutions. The American Journal of Medicine 1996;100(1A):62s‐69s. - PubMed
References to studies excluded from this review
Bauer 1975 {published data only}
-
- Bauer VP, Kummer F. The action of a tropic acid ester on bronchospasm:A double‐blind study [Zur wirkung eines tropasaure‐esters auf den bronchospasmus]. Wiener Klinische Wochenschrift 1975;87(4):132‐6. - PubMed
Brown 1984 {published data only}
Dejaegher 1984 {published data only}
-
- Dejaegher Ph, Demedts M, Rochette F, Veken J. Effects of 40ug and 400ug of ipratropium bromide in obstructive lung disease. Acta Clinica Belgica 1984;39(3):136‐40. - PubMed
Disse 1999 {published data only}
-
- Disse B, Speck GA, Rominger KL, Witek TJ, Hammer R. Tiotropium (SPIRIVA): Mechanistical considerations and clinical profile in obstructive lung disease. Life Sciences 1999;64(6/7):457‐64. - PubMed
Heimer 1991 {published data only}
-
- Heimer D, Brami JL, Liberman D, Bark H. Comparison of a B2 adrenergic agonist and an anticholinergic agent given by sequential inhalation in patients with severe chronic obstructive pulmonary disease. Israel Journal of Medical Science 1991;27:307‐10. - PubMed
Hidalgo 1983 {published data only}
-
- Banos Hidalgo P, Ramos Martos A, Cabrera Moreno R, Luque Leal J, Palcios Giner YA. Comparacion en estudio doble ciego de la asociacion de un aerosol de salbutamol y bromuro de ipratropio y de su efecto individual en bronquitis cronica y asma bronquial. Revista Clinica Espanola 1983;171(4):265‐8. - PubMed
Kheir 1993 {published data only}
-
- Kheir A, Ying Y, Hannhart B, Duvivier C, Peslin R, Polu JM. Comparison de l'effet bronchodilatateur at du site d'action du fenoterol et de l'ipratropium chez bronchiteux chroniques obstructifs [A comparison of the bronchodilator effect and the site of action of fenoterol and of ipratropium in bronchitis with airflow obstruction]. Revue des Maladies Respiratoires 1993;10:9‐15. - PubMed
Khristoliubova 1999 {published data only}
-
- Khristoliubova EI, Volkova LI, Shcherbakova IV. Long‐term atrovent treatment of chronic obstructive bronchitis. Klinicheskaia Meditsina (Mosk) 1999;77(12):51‐2. - PubMed
Lees 1980 {published data only}
-
- Lees AW, Allan GW, Smith J. Nebulised ipratropium bromide and salbutamol in chronic bronchitis. British Journal of Clinical Practice 1980;34(11‐12):340‐2. - PubMed
Leitch 1978 {published data only}
Lien 1980 {published data only}
-
- Lien J Th. Klinisk sammenligning av Atrovent og placebo hos pasienter med kronisk ostruktiv lungesykdom. Idsskr Nor Loegeforen 1980;100:1193‐5. - PubMed
Matera 1996 {published data only}
-
- Matera MG, Caputi M, Cazzola M. A combination with clinical recommended dosages of salmeterol and ipratropium is not more effective than salmeterol alone in patients with chronic obstructive pulmonary disease. Respiratory Medicine 1996;90:497‐9. - PubMed
Nardini 1996 {published data only}
-
- Nardini S. Inhaled antimuscarinic agents and COPD. Monaldi Archives for Chest Disease 1996;51(1):52‐3. - PubMed
Nishimura 1992 {published data only}
-
- Nishimura K, Koyama H, Izumi T. A comparison of bronchodilating drugs for the treatment of stable COPD. Nihon Hyobu Shikkan Gakkai Zasshi 1992;30(5):835‐43. - PubMed
Petro 1981 {published data only}
-
- Petro W, Nakhosteen JA, Konietzko N. Bronchodilator effect of a combination of a beta‐adrenergic agonist and a cholinergic antagonist [Broncholyse unter kombinierter anwendung von Beta‐adrenergen agonisten und cholinergen antagonisten]. Prax Pneumol 1981;35:779‐82.
Pierce 1982 {published data only}
-
- Pierce RJ, Holmes PW, Campbell AH. Use of ipratropium bromide in pateints with severe airways obstruction. Australian and New Zealand Journal of Medicine 1982;12:38‐43. - PubMed
Shmelev 1999 {published data only}
-
- Shmelev EI, Khmel'kova NG, Nonikov DV, Melent'eva EM, Abubikirov AF, Makarova VL, et al. Experience in long‐term use of berodual in the treatment of patients with chronic obstructive bronchitis. Terapevticheskii Arkhiv 1999;71(3):22‐4. - PubMed
Simanenkov 1998 {published data only}
-
- Simanenkov VI, Il'iashevich IG, Liparteliani BG, Ledovaia AV. Long‐term atrovent treatment of chronic obstructive bronchitis [Dlitel'noe lechenie atroventom pri khronicheskom obstruktivnom bronkhite]. Terapevticheskii Arkhiv 1998;70(9):77‐9. - PubMed
Tang 1984 {published data only}
-
- Tang OT, Flatley M. A comparison of effects of inhaling a combined preparation of fenoterol with ipratropium bromide (Duovent) with those of fenoterol and salbutamol. Postgraduate Medical Journal 1984;60:24‐7. - PubMed
Additional references
Anthonisen 1986
-
- Anthonisen NR, Wright EC. Bronchodilator response in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1986;133:814‐9. - PubMed
Anthonisen 1994
-
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: The Lung Health Study. JAMA 1994;272:1497‐505. - PubMed
Appleton 1999
-
- Appleton S, Smith B, Veale A, Bara A. Regular long‐acting beta‐2 adrenoceptor agonists for chronic obstructive pulmonary disease (Cochrane review). The Cochrane Database of Systematic Reviews 1999, Issue 4.
Appleton 2006
ATS 1995
-
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1995;152:s78‐s121. - PubMed
ATS/ERS 2004
-
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal 2004;23:932‐46. - PubMed
Barnes 1993
-
- Barnes PJ. Theoretical aspects of anticholinergic treatment. In: Gross NJ editor(s). Anticholinergic therapy in obstructive airways disease. London: Franlkin Scientific Publications, 1993.
Barr 2005
Belman 1996
-
- Belman M, Botnick W, Shin J. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1996;153:967‐75. - PubMed
Blosser 1995
-
- Blosser SA, Maxwell SL, Reeves‐Hoche MK, Localio AR, Zwillich CW. Is an anti‐cholinergic agent superior to a beta‐2 agonist in improving dyspnea and exercise limitation in COPD?. Chest 1995;108:730‐5. - PubMed
Boyd 1997
-
- Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1997;10:815‐21. - PubMed
BTS 1997
-
- British Thoracic Society. Chronic Obstructive Pulmonary Disease Guidelines. Thorax 1997;52(Suppl 5):S2‐S28.
Calverly 2003
Chapman 1991
-
- Chapman KR. Clinical implications of anticholinergic bronchodilator therapy in COPD. Res Clin Forums 1991;13(2):43‐50.
Cwealth of Aust 2000
-
- Commonwealth of Australia. Schedule of Pharmaceutical Benefits Effective from May 2001; GENERAL Respiratory system. http://www.health.gov.au/pbs/scripts/dispgp.cfm 2000.
D'Urzo 2001
-
- D'Urzo A, Salvo M, Ramirez‐Rivera A, Almeida J, Sichletidis L, Rapatz G, Kottakis J. In patients with COPD, treatment with a combination of formoterol and ipratropium is more effective than a combination of salbutamol and ipratropium: A 3 week, randomized, double‐blind, with‐in patient, multicentre study. Chest 2001;119:1347‐56. - PubMed
Ferguson 2000
-
- Ferguson G. Recommendations for the management of COPD. Chest 2000;117(2 Suppl):23s‐28s. - PubMed
Friedman 1999
-
- Friedman M, Serby C, Menjoge S, Wilson D, Hilleman D, Witek T. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone. Chest 1999;115:635‐41. - PubMed
GOLD 2003
-
- National Heart, Lung, and Blood Institute. [Global Initiative on Obstructive Lung Disease (GOLD) Pocket Guide (updated 2003)]. www.goldcopd.com [accessed 16/02/04].
GOLD 2004
-
- National Heart, Lung, and Blood Institute. Global Initiative for Chronic Obstructive Pulmonary Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (Updated 2004). www.goldcopd.com [accessed 21/03/05].
Jadad 1996
-
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomised controlled trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Lipworth 1989
Mahler 2002
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2002;166:1084‐91. - PubMed
Matera 1995
-
- Matera MG, Cazzola M, Vinciguerraaa A, et al. Comparison of the bronchodilating effects of salmeterol, salbutamol and ipratropium bromide in patients with chronic obstructive pulmonary disease. Pulmonary Pharmacology 1995;8:267‐71. - PubMed
NICE/BTS 2004
Nisar 1992
-
- Nisar M, Earis JE, Pearson MG, Calverly PMA. Acute bronchodilator trials in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1992;146:555‐9. - PubMed
O'Donnell 1997
-
- O'Donnell D, Bertley J, Chau L, Webb K. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiological mechanisms. American Journal of Respiratory & Critical Care Medicine 1997;155:109‐15. - PubMed
O'Donnell 1998
-
- O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation and endurance during exercise in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1998;158:1557‐65. - PubMed
O'Donnell 1999
-
- O' Donnell D, Lam M, Webb K. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1999;160:542‐9. - PubMed
O'Donnell 2000
-
- O' Donnell D. Assessment of bronchodilator efficacy in symptomatic COPD. Is spirometry useful?. Chest 2000;117(2 Suppl):42s‐47s. - PubMed
Pauwels 2000
-
- Pauwels R. National and international guidelines for COPD: The need for evidence. Chest 2000;117(2 Suppl):20s‐22s. - PubMed
Redelmeier 1996
-
- Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. Journal of Clinical Epidemiology 1996;49(11):1215‐19. - PubMed
Rossi 2002
-
- Rossi A, Kristufek P, Levine BE, Thomson MH, Till D, Kottakis J, et al. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow‐release theophylline in the treatment of COPD. Chest 2002;121:1058‐69. - PubMed
Snider 1985
-
- Snider GL, Klenerman J, Thurlbeck WM, Bengali ZH. The definition of emphysema: Report of a National Heart and Blood Institute, Division of Lung Disease, Workshop. American Review of Respiratory Disease 1985;132:182‐5. - PubMed
Spencer 2004
-
- Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. European Respiratory Journal 2004;23(5):698‐702. - PubMed
Tashkin 2003
-
- Tashkin D, Kesten S. Long‐term treatment benefits with tiotropium in COPD patients with and without short‐term bronchodilator responses. Chest 2003;123:1441‐49. - PubMed
Ullah 1981
Ulrik 1995
van Schayck 1991
van Weel 1998
-
- Weel C. Combining nebulised ipratropium and albuterol improved FEV1 but did not improve symptoms or quality of life in COPD. Evidence‐Based Medicine 1998:120.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

