Vaccines for preventing malaria (SPf66)
- PMID: 16625647
- PMCID: PMC6532709
- DOI: 10.1002/14651858.CD005966
Vaccines for preventing malaria (SPf66)
Abstract
Background: A malaria vaccine is badly needed. SPf66 was one of the earliest vaccines developed. It is a synthetic peptide vaccine containing antigens from the blood stages of malaria linked together with an antigen from the sporozoite stage, and is targeted mainly against the blood (asexual) stages.
Objectives: To assess the effect of SPf66 malaria vaccines against Plasmodium falciparum, P. vivax, P. malariae, and P. ovale in preventing infection, disease, and death.
Search strategy: We searched the Cochrane Infectious Diseases Group Specialized Register (September 2005), CENTRAL (The Cochrane Library 2005, Issue 3), MEDLINE (1966 to September 2005), EMBASE (1980 to September 2005), LILACS (1982 to September 2005), Science Citation Index (1981 to September 2005), and reference lists of articles. We also contacted organizations and researchers in the field.
Selection criteria: Randomized and quasi-randomized controlled trials comparing SPf66 vaccine with placebo or routine antimalarial control measures in people of any age receiving an artificial challenge or natural exposure to malaria infection (any species).
Data collection and analysis: Two people independently assessed trial quality and extracted data, including adverse events. Results were expressed as relative risks (RR) with 95% confidence intervals (CI).
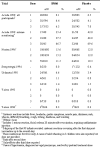
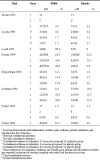
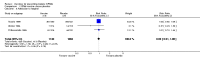
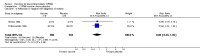
Main results: Ten efficacy trials of SPf66 involving 9698 participants were included. Results with SPf66 in reducing new episodes of P. falciparum malaria were heterogeneous: it was not effective in four African trials (RR 0.98, 95% CI 0.90 to 1.07; 2371 participants) or in one Asian trial (RR 1.06, 95% CI 0.90 to 1.25; 1221 participants). In four trials in South America the number of first attacks with P. falciparum was reduced by 28% (RR 0.72, 95% CI 0.63 to 0.82; 3807 participants). It did not reduce episodes of P. vivax malaria or admission to hospital with severe malaria. Trials have not indicated any serious adverse events with SPf66 vaccine.
Authors' conclusions: There is no evidence for protection by SPf66 vaccines against P. falciparum in Africa. There is a modest reduction in attacks of P. falciparum malaria following vaccination with SPf66 in South America. There is no justification for further trials of SPf66 in its current formulation. Further research with SPf66 vaccines in South America or with new formulations of SPf66 may be justified.
Conflict of interest statement
None known.
Figures






References
References to studies included in this review
Acosta 1999 {published data only}
-
- Acosta CJ, Galindo CM, Schellenberg D, Aponte JJ, Kahigwa E, Urassa H, et al. Evaluation of the SPf66 vaccine for malaria control when delivered through the EPI scheme in Tanzania. Tropical Medicine and International Health 1999;4(5):368‐76. - PubMed
-
- Galindo CM, Acosta CJ, Schellenberg D, Aponte JJ, Roca A, Oettli A, et al. Humoral immune responses during a malaria vaccine trial in Tanzanian infants. Parasite Immunology 2000;22(9):437‐43. - PubMed
-
- Schellenberg DM, Acosta CJ, Galindo CM, Kahigwa E, Urassa H, Masanja H, et al. Safety in infants of SPf66, a synthetic malaria vaccine, delivered alongside the EPI. Tropical Medicine and International Health 1999;4(5):377‐82. - PubMed
Alonso 1994 {published and unpublished data}
-
- Alonso PL, Lopez MC, Bordmann G, Smith TA, Aponte JJ, Weiss NA, et al. Immune responses to Plasmosium falciparum antigens during a malaria vaccine trial in Tanzanian children. Parasite Immunology 1998;20(2):63‐71. - PubMed
-
- Alonso PL, Smith T, Schellenberg JR, Masanja H, Mwankusye S, Urassa H, et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet 1994;344(8931):1175‐81. - PubMed
-
- Alonso PL, Smith TA, Armstrong‐Schellenberg JR, Kitua AY, Masanja H, Hayes R, et al. Duration of protection and age‐dependence of the effects of the SPf66 malaria vaccine in African children exposed to intense transmission of Plasmodium falciparum. Journal of Infectious Diseases 1996;174(2):367‐72. - PubMed
-
- Alonso PL, Tanner M, Smith T, Hayes RJ, Schellenberg JA, Lopez MC, et al. A trial of the synthetic malaria vaccine SPf66 in Tanzania: rationale and design. Vaccine 1994;12(2):181‐6. - PubMed
-
- Alonzo PL, Smith T, Schellenberg JR, Masanja H, Mwankusye S, Urassa H, et al. Randomized trial of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Medicine Tropicale 1995;55 Suppl:41‐6. - PubMed
D'Alessandro 1995 {published data only}
-
- Bojang KA, Obaro SK, D'Alessandro U, Bennett S, Langerock P, Targett GA, et al. An efficacy trial of the malaria vaccine SPf66 in Gambian infants ‐ second year of follow‐up. Vaccine 1998;16(1):62‐7. - PubMed
-
- D'Alessandro U. An efficacy trial of a malaria vaccine in Gambian infants and comparison with insecticide treated bednets. Annals of Tropical Medicine and Parasitology 1996;90(4):373‐8. - PubMed
-
- D'Alessandro U, Leach A, Drakeley CJ, Bennett S, Olaleye BO, Fegan GW, et al. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet 1995;346(8973):462‐7. - PubMed
-
- Haywood M, Conway DJ, Weiss H, Metzger W, D'Alessandro U, Snounou G, et al. Reduction in the mean number of Plasmodium falciparum genotypes in Gambian children immunized with the malaria vaccine SPf66. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93 Suppl 1:65‐8. - PubMed
-
- Metzger WG, Haywood M, D'Alessandro U, Drakeley CJ, Weiss H, Bojang K, et al. Serological responses of Gambian children to immunization with the malaria vaccine SPf66. Parasite Immunology 1999;21(7):335‐40. - PubMed
Leach 1995 {published data only}
-
- Bojang KA, Obaro SK, Leach A, D'Alessandro U, Bennett S, Metzger W, et al. Follow‐up of Gambian children recruited to a pilot safety and immunogenicity study of the malaria vaccine SPf66. Parasite Immunology 1997;19(12):579‐81. - PubMed
-
- Leach A, Drakeley C, D'Alessandro U, Fegan G, Bennett S, Ballou WR, et al. A pilot safety and immunogenicity study of the malaria vaccine SPf66 in Gambian infants. Parasite Immunology 1995;17(8):441‐4. - PubMed
Masinde 1998 {published and unpublished data}
-
- Duffy PE, Ogutu BR, Nyakeriga AM, Opollo MO, Ohas EA, Opiyo M, et al. A phase 1 trial comparing subcutaneous and intramuscular delivery of SPf66: reactogenicity and immunogenicity. The 45th Annual Meeting of the American Society of Tropical Medicine and Hygiene. Baltimore, Maryland, December 1‐5, 1996 1996;55 Suppl 2:132.
-
- Masinde GL, Krogstad DJ, Gordon DM, Duffy PE. Immunization with SPf66 and subsequent infection with homologous and heterologous Plasmodium falciparum parasites. American Journal of Tropical Medicine and Hygiene 1998;59(4):600‐5. - PubMed
Nosten 1996 {published data only}
-
- Ballou WR, Blood JC, Chongsuphajaissidhi T, Gordon DM, Heppner DG, Kyle DE, et al. Field trials of an asexual blood stage vaccine: studies of the synthetic peptide polymer SPf66 in Thailand and the analytic plan for a phase IIb efficacy study. Parasitology 1995;110 Suppl:25‐36. - PubMed
-
- Nosten F, Luxemburger C, Kyle DE, Ballou WR, Wittes J, Wah E, et al. Randomised double‐blind placebo‐controlled trial of SPf66 malaria vaccine in children in northwestern Thailand. Lancet 1996;348(9029):701‐7. - PubMed
Sempertegui 1994 {published data only}
-
- Sempertegui F, Estrella B, Moscoso J, Piedrahita L, Hernandez D, Gaybor J, et al. Safety, immunogenicity and protective effect of the SPf66 malaria synthetic vaccine against Plasmodium falciparum infection in a randomized double‐blind placebo‐controlled field trial in an endemic area of Ecuador. Vaccine 1994;12(4):337‐42. - PubMed
Urdaneta 1998 {published data only}
-
- Urdaneta M, Prata A, Struchiner CJ, Tosta CE, Tauil P, Boulos M. Evaluation of SPf66 malaria vaccine efficacy in Brazil. American Journal of Tropical Medicine and Hygiene 1998;58(3):378‐85. - PubMed
-
- Urdaneta M, Prata A, Struchiner CJ, Tosta CE, Tauil P, Boulos M. SPf66 vaccine trial in Brazil: conceptual framework, study design and analytical approach. Revista da Sociedade Brasileira de Medicina Tropical 1996;29(3):259‐69. - PubMed
-
- Urdaneta M, Prata A, Struchiner CJ, Tosta CE, Tauil P, Boulos M. Safety evaluation of SPf66 vaccine in Brazil. Revista da Sociedade Brasileira de Medicina Tropical 1996;29(5):497‐501. - PubMed
Valero 1993 {published data only}
-
- Valero MV, Amador A, Galindo C, Figueroa J, Bello MS, Murillo LA, et al. Vaccination with SPf66, a chemically synthesised vaccine, against Plasmodium falciparum in Colombia. Lancet 1993;341(8847):705‐10. - PubMed
Valero 1996 {published data only}
-
- Valero MV, Amador R, Aponte JJ, Narvaez A, Galindo C, Silva Y, et al. Evaluation of SPf66 malaria vaccine during a 22‐month follow‐up field trial in the Pacific coast of Colombia. Vaccine 1996;14(15):1466‐70. - PubMed
References to studies excluded from this review
Amador 1992 {published data only}
-
- Amador R, Moreno A, Valero V, Murillo L, Mora AL, Rojas M, et al. The first field trials of the chemically synthesized malaria vaccine SPf66: safety, immunogenicity and protectivity. Vaccine 1992;10(3):179‐84. - PubMed
Gordon 1996 {published data only}
-
- Gordon DM, Duffy PE, Heppner DG, Lyon JA, Williams JS, Scheumann D, et al. Phase I safety and immunogenicity testing of clinical lots of the synthetic Plasmodium falciparum vaccine SPf66 produced under good manufacturing procedure conditions in the United States. American Journal of Tropical Medicine and Hygiene 1996;55(1):63‐8. - PubMed
Migasena 1997 {published data only}
-
- Migasena S, Heppner DG, Kyle DE, Chongsuphajaisiddhi T, Gordon DM, Suntharasamai P, et al. SPf66 malaria vaccine is safe and immunogenic in malaria naive adults in Thailand. Acta Tropica 1997;67(3):215‐27. - PubMed
Nosten 1997 {published data only}
-
- Nosten F, Luxemburger C, Kyle DE, Gordon DM, Ballou WR, Sadoff JC, et al. Phase I trial of the SPf66 malaria vaccine in a malaria‐experienced population in Southeast Asia. American Journal of Tropical Medicine and Hygiene 1997;56(5):526‐32. - PubMed
Noya 1994 {published data only}
-
- Noya O, Gabaldon Berti Y, Alarcon de Noya B, Borges R, Zerpa N, Urbaez JD, et al. A population‐based clinical trial with the SPf66 synthetic Plasmodium falciparum malaria vaccine in Venezuela. Journal of Infectious Diseases 1994;170(2):396‐402. - PubMed
Patarroyo 1988 {published data only}
-
- Patarroyo ME, Amador R, Clavijo P, Moreno A, Guzman F, Romero P, et al. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature 1988;332(6160):158‐61. - PubMed
Patarroyo 1992 {published data only}
-
- Patarroyo G, Franco L, Amador R, Murillo LA, Rocha CL, Rojas M, et al. Study of the safety and immunogenicity of the synthetic malaria SPf66 vaccine in children aged 1‐14 years. Vaccine 1992;10(3):175‐8. - PubMed
Additional references
Ballou 2004
-
- Ballou WR, Arrevalo‐Herrera M, Carucci D, Richie TL, Corradin G, Diggs C, et al. Update on the clinical development of candidate malaria vaccines. American Journal of Tropical Medicine and Hygiene 2004;71 Suppl 2:239‐47. - PubMed
Breman 2004
-
- Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. American Journal of Tropical Medicine and Hygiene 2004;71 Suppl 2:1‐15. - PubMed
Graves 2006a
Graves 2006b
Hay 2004
Higgins 2005
-
- Higgins J, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 1 September 2005).
Juni 2001
Kashala 2002
-
- Kashala O, Amador R, Valero MV, Moreno A, Barbaosa A, Nickel B, et al. Safety, tolerability and immunogenicity of new formulations of the Plasmodium falciparum malaria peptide vaccine SPf66 combined with the immunological adjuvant QS‐21. Vaccine 2002;20(17‐8):2263‐77. - PubMed
Review Manager 4.2 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Richie 2002
-
- Richie TL, Saul A. Progress and challenges for malaria vaccines. Nature 2002;415(6872):694‐701. - PubMed
Snow 2004
-
- Snow RW, Korenromp EL, Gouws E. Pediatric mortality in Africa: Plasmodium falciparum as a cause or risk?. American Journal of Tropical Medicine and Hygiene 2004;71(Suppl 2):16‐24. - PubMed
Snow 2005
WHO 2002
-
- World Health Organization. The world health report 2002: reducing risks, promoting healthy life. Geneva: World Health Organization, 2002. - PubMed
References to other published versions of this review
Graves 1998
-
- Graves P, Gelband H, Garner P. The SPf66 malaria vaccine: what is the evidence for efficacy?. Parasitology Today 1998;14:218‐20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

