Adventures in vascular biology: a tale of two mediators
- PMID: 16627292
- PMCID: PMC1609404
- DOI: 10.1098/rstb.2005.1775
Adventures in vascular biology: a tale of two mediators
Abstract
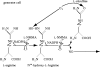
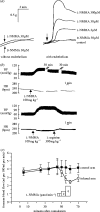
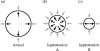
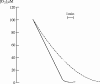
I would like to thank the Royal Society for inviting me to deliver the Croonian Lecture. In so doing, the Society is adding my name to a list of very distinguished scientists who, since 1738, have preceded me in this task. This is, indeed, a great honour. For most of my research career my main interest has been the understanding of the normal functioning of the blood vessel wall and the way this is affected in pathology. During this time, our knowledge of these subjects has grown to such an extent that many people now believe that the conquering of vascular disease is a real possibility in the foreseeable future. My lecture concerns the discovery of two substances, prostacyclin and nitric oxide. I would like to describe the moments of insight and some of the critical experiments that contributed significantly to the uncovering of their roles in vascular biology. The process was often adventurous, hence the title of this lecture. It is the excitement of the adventure that I would like to convey in the text that follows.
Figures

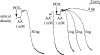


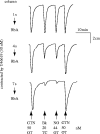
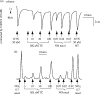
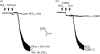
References
-
- Akarasereenont P, Techatraisak K, Thaworn A, Chotewuttakorn S. The induction of cyclooxygenase-2 by 17beta-estradiol in endothelial cells is mediated through protein kinase C. Inflamm. Res. 2000;49:460–465. 10.1007/s000110050617 - DOI - PubMed
-
- Alderton W.K, Cooper C.E, Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. 10.1042/0264-6021:3570593 - DOI - PMC - PubMed
-
- Almeida A, Moncada S, Bolanos J.P. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 2004;6:45–51. 10.1038/ncb1080 - DOI - PubMed
-
- Amezcua J.L, O'Grady J, Salmon J.A, Moncada S. Prolonged paradoxical effect of aspirin on platelet behaviour and bleeding time in man. Thromb. Res. 1979;16:69–79. 10.1016/0049-3848(79)90270-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
