Ovulation, pregnancy, placentation and husbandry in the African elephant (Loxodonta africana)
- PMID: 16627297
- PMCID: PMC1609400
- DOI: 10.1098/rstb.2006.1831
Ovulation, pregnancy, placentation and husbandry in the African elephant (Loxodonta africana)
Abstract
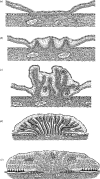
The African elephant reproduces so efficiently in the wild that overpopulation is now a serious problem in some game parks in Zimbabwe, Botswana and South Africa. The female reaches puberty between 10 and 12 years of age in the wild and, when in captivity, shows oestrous cycles of 14-15 weeks duration. She readily conceives a singleton in the wild yet her uterus has the capacity for twins. She shows a gestation length of 22 months and, in the wild, shows a population density and feed dependent intercalving interval of 4-8 years. The trophoblast erodes the lumenal epithelium of the endometrium and stimulates upgrowths of blood vessel-containing stromal villi, which develop eventually into the broad, tightly folded lamellae of the zonary, endotheliochorial placenta. Significant quantities of leaked maternal erythrocytes and ferric iron are phagocytosed by specialized trophoblast cells in the haemophagous zones at the lateral edges of the placental band. Although the placenta itself is endocrinologically inert, the foetal gonads, which enlarge greatly during the second half of pregnancy can synthesize 5alpha-dihydryoprogesterone and other 5alpha pregnane derivatives from cholesterol and pregnenolone. These products may synergize with progestagens secreted by the 2-8 large corpora lutea which are always present in the maternal ovaries throughout gestation to maintain the pregnancy state.
Figures



References
-
- Adams C.E. Embryo transfer in rodents. In: Adams C.E, editor. Mammalian egg transfer. CRC Press; Boca Raton, FL: 1982. pp. 22–23.
-
- Allen W.R, Mathias S.S, Wooding F.B.P, Skidmore J.A, van Aarde R.J. Placentation in the African elephant, Loxodonta africana. I. Endocrinological aspects. Reproduction. 2002a;60:105–116. - PubMed
-
- Allen W.R, Wilsher S, Stewart F, Ousey J, Fowden A. The influence of maternal size on placental, fetal and postnatal growth in the horse. II Endocrinology of pregnancy. J. Endocrinol. 2002b;172:237–246. 10.1677/joe.0.1720237 - DOI - PubMed
-
- Allen W.R, Mathias S, Wooding F.B.P, van Aarde R.J. Placentation in the African elephant (Loxodonta africana). II. Morphological changes in the uterus and placenta throughout gestation. Placenta. 2003;24:598–617. 10.1016/S0143-4004(03)00102-4 - DOI - PubMed
-
- Allen W.R, Stout S, Ford M. Placentation in the African elephant, Loxodonta africana. IV. Growth and organisation of the fetal gonads. Reproduction. 2005;130:713–720. 10.1530/rep.1.00696 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
