Proprotein convertase furin is preferentially expressed in T helper 1 cells and regulates interferon gamma
- PMID: 16627761
- PMCID: PMC1895858
- DOI: 10.1182/blood-2005-09-3824
Proprotein convertase furin is preferentially expressed in T helper 1 cells and regulates interferon gamma
Abstract
Interleukin 12 (IL-12) is a major inducer of interferon gamma (IFN-gamma) and the principal mediator of T helper 1 (Th1) differentiation. To identify IL-12-regulated genes, which might contribute to Th1 differentiation and IFNG regulation, we employed microarray analysis. Surprisingly, a ubiquitously expressed proprotein convertase (PC), furin, was one of the most consistently IL-12-induced genes in T cells, and among PCs was the only one regulated by this cytokine. Furin was preferentially expressed in differentiated Th1 cells in a Stat4-dependent manner. Expression of furin enhanced IFN-gamma secretion, whereas inhibition of furin interfered with IFN-gamma production. Thus, we conclude that IL-12 induction of furin might represent a new aspect of IFN-gamma regulation and control of Th1 differentiation.
Figures
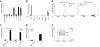

References
-
- Mattner F, Magram J, Ferrante J, et al. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26: 1553-1559. - PubMed
-
- Wu C, Ferrante J, Gately MK, Magram J. Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159: 1658-1665. - PubMed
-
- Huang S, Hendriks W, Althage A, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259: 1742-1745. - PubMed
-
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259: 1739-1742. - PubMed
-
- Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20: 581-620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

