Flk prevents premature secretion of the anti-sigma factor FlgM into the periplasm
- PMID: 16629666
- PMCID: PMC3471667
- DOI: 10.1111/j.1365-2958.2006.05135.x
Flk prevents premature secretion of the anti-sigma factor FlgM into the periplasm
Erratum in
- Mol Microbiol. 2006 Jul;61(1):269
Abstract
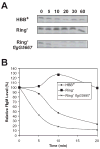
The flk locus of Salmonella typhimurium was identified as a regulator of flagellar gene expression in strains defective in P- and l-ring formation. Flk acts as a regulator of flagellar gene expression by modulating the protein levels of the anti-sigma28 factor FlgM. Evidence is presented which suggests that Flk is a cytoplasmic-facing protein anchored to the inner membrane by a single, C-terminal transmembrane-spanning domain (TMS). The specific amino acid sequence of the TMS is not essential for Flk activity, but membrane anchoring is essential. Membrane fractionation and visualization of protein fusions of green fluorescent protein derivatives to Flk suggested that the Flk protein is present in the membrane as punctate spots in number that are much greater than the number of flagellar basal structures. The turnover of the anti-sigma28 factor FlgM was increased in flk mutant strains. Using FlgM-beta-lactamase fusions we show the increased turnover of FlgM in flk null mutations is due to FlgM secretion into the periplasm where it is degraded. Our data suggest that Flk inhibits FlgM secretion by acting as a braking system for the flagellar-associated type III secretion system. A model is presented to explain a role for Flk in flagellar assembly and gene regulatory processes.
Figures



References
-
- Aizawa S-I. Flagellar assembly in Salmonella typhimurium. Mol Microbiol. 1996;20:1–4. - PubMed
-
- Aldridge P, Jenal U. Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Mol Microbiol. 1999;32:379–391. - PubMed
-
- Aldridge P, Karlinsey J, Hughes KT. The type III secretion chaperone FlgN regulates flagellar assembly via a negative feedback loop containing its chaperone substrates FlgK and FlgL. Mol Microbiol. 2003;49:1333–1345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

