Induction of centrosome amplification in p53 siRNA-treated human fibroblast cells by radiation exposure
- PMID: 16630116
- PMCID: PMC11159000
- DOI: 10.1111/j.1349-7006.2006.00168.x
Induction of centrosome amplification in p53 siRNA-treated human fibroblast cells by radiation exposure
Abstract
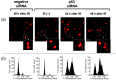
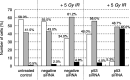
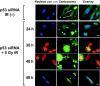
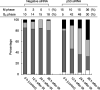
Centrosome amplification can be detected in the tissues of p53(-/-) mice. In contrast, loss of p53 does not induce centrosome amplification in cultured human cells. However, examination of human cancer tissues and cultured cells has revealed a significant correlation between loss or mutational inactivation of p53 and occurrence of centrosome amplification, supporting the notion that p53 mutation alone is insufficient to induce centrosome amplification in human cells, and that additional regulatory mechanisms are involved. It has recently been shown that gamma irradiation of tumor cells induces centrosome amplification. However, the precise mechanism of radiation-induced centrosome amplification is not fully understood. In the present study, CCD32SK diploid normal human fibroblasts were transfected transiently with short interfering RNA (siRNA) specific for human p53 (CCD/p53i). There was a small increase in the frequency of centrosome amplification in CCD/p53i cells (4.0%) without irradiation. In contrast, CCD/p53i cells after 5-Gy irradiation showed a marked increase in abnormal nuclear shapes and pronounced amplification of centrosomes (46.0%). At 12 h after irradiation, irradiated CCD/p53i cells were arrested in G(2) phase. By laser scanning cytometry, abnormal mitosis with amplified centrosomes was observed frequently in the accumulating G(2)/M population at 48 h after irradiation. In the present study, we found that siRNA-mediated silencing of p53 in normal human fibroblasts, together with DNA damage by irradiation, efficiently induced centrosome amplification and nuclear fragmentation, but these phenomena were not observed with either siRNA-mediated silencing of p53 or irradiation alone.
(Cancer Sci 2006; 97: 252-258).
Figures


Similar articles
-
Roles of cyclins A and E in induction of centrosome amplification in p53-compromised cells.Oncogene. 2008 Sep 11;27(40):5288-302. doi: 10.1038/onc.2008.161. Epub 2008 May 19. Oncogene. 2008. PMID: 18490919 Free PMC article.
-
Induction of centrosome amplification and chromosome instability in human bladder cancer cells by p53 mutation and cyclin E overexpression.Cancer Res. 2004 Jul 15;64(14):4800-9. doi: 10.1158/0008-5472.CAN-03-3908. Cancer Res. 2004. PMID: 15256449
-
Centrosome hyperamplification and chromosomal damage after exposure to radiation.Oncology. 2004;67(5-6):460-70. doi: 10.1159/000082931. Oncology. 2004. PMID: 15714003
-
Deregulation of the centrosome cycle and the origin of chromosomal instability in cancer.Adv Exp Med Biol. 2005;570:393-421. doi: 10.1007/1-4020-3764-3_14. Adv Exp Med Biol. 2005. PMID: 18727509 Review.
-
Centrosome amplification, chromosome instability and cancer development.Cancer Lett. 2005 Dec 8;230(1):6-19. doi: 10.1016/j.canlet.2004.12.028. Cancer Lett. 2005. PMID: 16253756 Review.
Cited by
-
The centrosome and cell proliferation.Cell Div. 2006 Nov 16;1:26. doi: 10.1186/1747-1028-1-26. Cell Div. 2006. PMID: 17109756 Free PMC article.
-
Roles of cyclins A and E in induction of centrosome amplification in p53-compromised cells.Oncogene. 2008 Sep 11;27(40):5288-302. doi: 10.1038/onc.2008.161. Epub 2008 May 19. Oncogene. 2008. PMID: 18490919 Free PMC article.
-
Differential regulation of centrosome integrity by DNA damage response proteins.Cell Cycle. 2008 Jul 15;7(14):2225-33. doi: 10.4161/cc.7.14.6303. Epub 2008 May 14. Cell Cycle. 2008. PMID: 18635967 Free PMC article.
-
Modulation of DNA Damage Response by Sphingolipid Signaling: An Interplay that Shapes Cell Fate.Int J Mol Sci. 2020 Jun 24;21(12):4481. doi: 10.3390/ijms21124481. Int J Mol Sci. 2020. PMID: 32599736 Free PMC article. Review.
-
P53, cyclin-dependent kinase and abnormal amplification of centrosomes.Biochim Biophys Acta. 2008 Sep;1786(1):15-23. doi: 10.1016/j.bbcan.2008.04.002. Epub 2008 Apr 22. Biochim Biophys Acta. 2008. PMID: 18472015 Free PMC article. Review.
References
-
- Brinkley BR. Microtubule organizing centers. Annu Rev Cell Biol 1985; 1: 145–72. - PubMed
-
- Lange BM, Faragher AJ, March P et al. Centriole duplication and maturation in animal cells. Curr Top Dev Biol 2000; 49: 235–49. - PubMed
-
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature 1997; 386: 623–7. - PubMed
-
- Brinkley BR, Goepfert TM. Supernumerary centrosomes and cancer: Boveri's hypothesis resurrected. Cell Motil Cytoskeleton 1998; 41: 281–8. - PubMed
-
- Kawamura K, Moriyama M, Shiba N et al. Centrosome hyperamplification and chromosomal instability in bladder cancer. Eur Urol 2003; 43: 505–15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

