Wilms' tumor gene WT1 17AA(-)/KTS(-) isoform induces morphological changes and promotes cell migration and invasion in vitro
- PMID: 16630117
- PMCID: PMC11160036
- DOI: 10.1111/j.1349-7006.2006.00169.x
Wilms' tumor gene WT1 17AA(-)/KTS(-) isoform induces morphological changes and promotes cell migration and invasion in vitro
Abstract
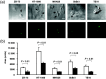
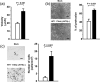
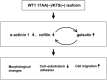
The wild-type Wilms' tumor gene WT1 is overexpressed in human primary leukemia and in a wide variety of solid cancers. All of the four WT1 isoforms are expressed in primary cancers and each is considered to have a different function. However, the functions of each of the WT1 isoforms in cancer cells remain unclear. The present study demonstrated that constitutive expression of the WT1 17AA(-)/KTS(-) isoform induces morphological changes characterized by a small-sized cell shape in TYK-nu.CP-r (TYK) ovarian cancer cells. In the WT1 17AA(-)/KTS(-) isoform-transduced TYK cells, cell-substratum adhesion was suppressed, and cell migration and in vitro invasion were enhanced compared to that in mock vector-transduced TYK cells. Constitutive expression of the WT1 17AA(-)/KTS(-) isoform also induced morphological changes in five (one gastric, one esophageal, two breast and one fibrosarcoma) of eight cancer cell lines examined. No WT1 isoforms other than the WT1 17AA(-)/KTS(-) isoform induced the phenotypic changes. A decrease in alpha-actinin 1 and cofilin expression and an increase in gelsolin expression were observed in WT1 17AA(-)/KTS(-) isoform-transduced TYK cells. In contrast, co-expression of alpha-actinin 1 and cofilin or knockdown of gelsolin expression by small interfering RNA restored WT1 17AA(-)/KTS(-) isoform-transduced TYK cells to a phenotype that was comparable to that of the parent TYK cells. These results indicated that the WT1 17AA(-)/KTS(-) isoform exerted its oncogenic functions through modulation of cytoskeletal dynamics. The present results may provide a novel insight into the signaling pathway of the WT1 gene for its oncogenic functions.
(Cancer Sci 2006; 97: 259-270).
Figures





References
-
- Call KM, Glaser T, Ito CY et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 1990; 60: 509–20. - PubMed
-
- Harrington MA, Konicek B, Song A, Xia XL, Fredericks WJ, Rauscher FJ 3rd. Inhibition of colony‐stimulating factor‐1 promoter activity by the product of the Wilms’ tumor locus. J Biol Chem 1993; 268: 21 271–5. - PubMed
-
- Drummond IA, Madden SL, Rohwer‐Nutter P, Bell GI, Sukhatme VP, Rauscher FJ 3rd. Repression of the insulin‐like growth factor II gene by the Wilms tumor suppressor WT1. Science 1992; 257: 674–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

