Shock wave induced cytoskeletal and morphological deformations in a human renal carcinoma cell line
- PMID: 16630122
- PMCID: PMC11159309
- DOI: 10.1111/j.1349-7006.2006.00172.x
Shock wave induced cytoskeletal and morphological deformations in a human renal carcinoma cell line
Abstract
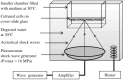
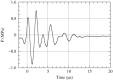
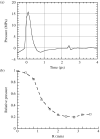
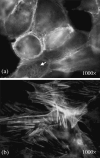
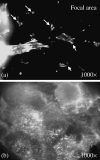
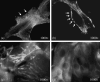
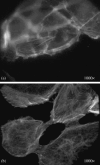
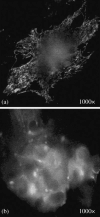
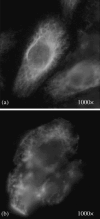
Effects of shock waves on the morphology and cytoskeleton of a human renal carcinoma cell line (ACHN) were investigated in vitro. ACHN monolayer cultured on a cover slide glass was treated with 10 shots of focused underwater shock waves, with 16 MPa peak pressure at the focal area of a piezoceramic shock wave generator. After exposure to the shock wave, based on the severity of morphological deformations of the treated cells, the monolayer was divided into three morphological areas; focal, marginal and intact. Morphological deformations were found to be associated with disorganization of the intracellular cytoskeletal filaments. Deformation of the cytoskeletal proteins in the treated cells were separately studied with respect to the location of the cells within the three morphological areas. Among three major cytoskeletal proteins, actin and tubulin, but not vimentin, were affected by the shock waves. The deformed cells reorganized their cytoskeletal network within 3 h with a pattern similar to the control, indicating the transient characteristic of the shock wave induced cytoskeletal damage in the surviving cells. The remaining cell fragments on the slide glass, which contained short actin filaments, indicated the important role of shear stress in damaging the cytoskeletal fibers by shock waves.
(Cancer Sci 2006; 97: 296-304).
Figures


Similar articles
-
A heat-shock-like response with cytoskeletal disruption occurs following hydrostatic pressure in MG-63 osteosarcoma cells.Biochem Cell Biol. 1993 Jul-Aug;71(7-8):361-71. doi: 10.1139/o93-054. Biochem Cell Biol. 1993. PMID: 7510113
-
Shiga toxin binding to globotriaosyl ceramide induces intracellular signals that mediate cytoskeleton remodeling in human renal carcinoma-derived cells.J Cell Sci. 2004 Aug 1;117(Pt 17):3911-22. doi: 10.1242/jcs.01246. Epub 2004 Jul 20. J Cell Sci. 2004. PMID: 15265987
-
Hydrostatic pressure has different effects on the assembly of tubulin, actin, myosin II, vinculin, talin, vimentin, and cytokeratin in mammalian tissue cells.Exp Cell Res. 1996 Sep 15;227(2):285-97. doi: 10.1006/excr.1996.0278. Exp Cell Res. 1996. PMID: 8831567
-
Cytoskeletal dynamics in dendritic spines: direct modulation by glutamate receptors?Trends Neurosci. 1999 Jul;22(7):290-5. doi: 10.1016/s0166-2236(99)01404-6. Trends Neurosci. 1999. PMID: 10370249 Review.
-
Human erythrocytes: cytoskeleton and its origin.Cell Mol Life Sci. 2020 May;77(9):1681-1694. doi: 10.1007/s00018-019-03346-4. Epub 2019 Oct 25. Cell Mol Life Sci. 2020. PMID: 31654099 Free PMC article. Review.
Cited by
-
Shock Waves Enhance Expression of Glycosphingolipid Tumor Antigen on Renal Cell Carcinoma: Dynamics of Physically Unmasking Hidden Intracellular Markers Independent of Gene-Signaling Pathways.Biomedicines. 2022 Feb 24;10(3):545. doi: 10.3390/biomedicines10030545. Biomedicines. 2022. PMID: 35327347 Free PMC article.
-
Impact of extracorporeal shock waves on the human skin with cellulite: a case study of an unique instance.Clin Interv Aging. 2008;3(1):201-10. doi: 10.2147/cia.s2334. Clin Interv Aging. 2008. PMID: 18488890 Free PMC article.
-
The effect of low intensity shockwave treatment (Li-SWT) on human myoblasts and mouse skeletal muscle.BMC Musculoskelet Disord. 2017 Dec 29;18(1):557. doi: 10.1186/s12891-017-1879-4. BMC Musculoskelet Disord. 2017. PMID: 29284454 Free PMC article.
-
RhoA and Rac1 as Mechanotransduction Mediators in Colorectal Cancer.Adv Biol (Weinh). 2025 Aug;9(8):e2400626. doi: 10.1002/adbi.202400626. Epub 2025 Jan 30. Adv Biol (Weinh). 2025. PMID: 39887960 Free PMC article.
-
Focused shock waves and inertial cavitation release tumor-associated antigens from renal cell carcinoma.Ultrason Sonochem. 2024 Dec;111:107078. doi: 10.1016/j.ultsonch.2024.107078. Epub 2024 Sep 20. Ultrason Sonochem. 2024. PMID: 39327122 Free PMC article.
References
-
- Oosterhof GON, Smits GA, Hj deRuyter JE et al. The in vitro effect of electromagnetically generated shock waves (Lithostar) on the Dunning R3327 PAT‐2 rat prostatic cancer cell‐line. Urol Res 1989; 17: 13–19. - PubMed
-
- Russo P, Stephenson RA, Mies C et al. High energy shock waves suppress tumor growth in vitro and in vivo. J Urol 1986; 135: 626–8. - PubMed
-
- Brauner T, Brummer F, Hulser DF. Histopathology of shock wave treated tumor cell suspensions and multicell tumor spheroids. Ultrasound Med Biol 1989; 15: 451–60. - PubMed
-
- Brummer F, Brenner J, Brauner T, Hulser DF. Effect of shock waves on suspension and immobilized L1210 cells. Ultrasound Med Biol 1989; 15: 229–39. - PubMed
-
- Endl E, Steinbach P, Scharfe J, Fickweiler S, Worle K, Hofstadter F. Cell‐type‐specific response to shock waves of suspended or pelleted cells as analyzed by flow cytometry or electrical cell volume determination. Ultrasound Med Biol 1996; 22: 515–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

