Identification of secernin 1 as a novel immunotherapy target for gastric cancer using the expression profiles of cDNA microarray
- PMID: 16630140
- PMCID: PMC11159625
- DOI: 10.1111/j.1349-7006.2006.00194.x
Identification of secernin 1 as a novel immunotherapy target for gastric cancer using the expression profiles of cDNA microarray
Abstract
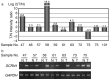
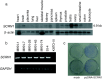
Despite the discovery of multiple TAAs, only a limited number is available for clinical application, particularly against epithelial malignancies. In this study we searched for novel TAAs using expression profiles of gastric cancer examined with cDNA microarray, and identified the SCRN1 gene as a candidate. SCRN1 was confirmed to be expressed in five out of seven gastric cancers with semiquantitative RT-PCR. With Northern blot analysis, it was detected abundantly in the testis and ovary, but it was barely detectable in 14 other normal human adult organs. Colony formation assay revealed that its augmented expression is associated with promoted cell growth. As these expression profiles and functional features of SCRN1 appeared to be compatible with the characteristics of the hypothesized ideal TAAs, we examined whether SCRN1 protein contains antigenic epitope peptides restricted to HLA-A*0201. We synthesized the candidate peptides derived from SCRN1, and tried to induce CTLs with each peptide. The CTL clones were successfully induced with a peptide SCRN1-196 (KMDAEHPEL), and they lyzed not only the peptide-pulsed targets but also the tumor cells expressing both SCRN1 and HLA-A*0201 endogenously. These results strongly suggest that SCRN1-196 is an epitope peptide restricted to HLA-A*0201. Furthermore, we synthesized an anchor-modified peptide SCRN1-9 V (KMDAEHPEV), in which leucine at position 9 was substituted for valine to increase the binding affinity to the HLA-A*0201 molecules. The CTL clones induced by SCRN1-9 V also recognized tumor cells expressing its natural SCRN1 protein endogenously. These results strongly suggest that SCRN1 is a novel TAA and these peptides, both native and modified, may be applicable for cancer vaccines to treat gastric cancer.
Figures


Similar articles
-
Identification of a novel tumor-associated antigen, cadherin 3/P-cadherin, as a possible target for immunotherapy of pancreatic, gastric, and colorectal cancers.Clin Cancer Res. 2008 Oct 15;14(20):6487-95. doi: 10.1158/1078-0432.CCR-08-1086. Clin Cancer Res. 2008. PMID: 18927288
-
Ring finger protein 43 as a new target for cancer immunotherapy.Clin Cancer Res. 2004 Dec 15;10(24):8577-86. doi: 10.1158/1078-0432.CCR-04-0104. Clin Cancer Res. 2004. PMID: 15623641
-
A novel tumor-associated antigen, cell division cycle 45-like can induce cytotoxic T-lymphocytes reactive to tumor cells.Cancer Sci. 2011 Apr;102(4):697-705. doi: 10.1111/j.1349-7006.2011.01865.x. Epub 2011 Feb 10. Cancer Sci. 2011. PMID: 21231984
-
HLA-A2-restricted CTL epitopes of a novel lung cancer-associated cancer testis antigen, cell division cycle associated 1, can induce tumor-reactive CTL.Int J Cancer. 2008 Dec 1;123(11):2616-25. doi: 10.1002/ijc.23823. Int J Cancer. 2008. PMID: 18770861
-
Cancer immunotherapy using novel tumor-associated antigenic peptides identified by genome-wide cDNA microarray analyses.Cancer Sci. 2015 May;106(5):505-11. doi: 10.1111/cas.12650. Epub 2015 Apr 1. Cancer Sci. 2015. PMID: 25726868 Free PMC article. Review.
Cited by
-
Expression analysis of Barrett's esophagus-associated high-grade dysplasia in laser capture microdissected archival tissue.Clin Cancer Res. 2008 Oct 15;14(20):6440-8. doi: 10.1158/1078-0432.CCR-08-0302. Clin Cancer Res. 2008. PMID: 18927283 Free PMC article.
-
Identification of genes specifically methylated in Epstein-Barr virus-associated gastric carcinomas.Cancer Sci. 2013 Oct;104(10):1309-14. doi: 10.1111/cas.12228. Epub 2013 Aug 9. Cancer Sci. 2013. PMID: 23829175 Free PMC article.
-
Knockdown of Secernin 1 inhibit cell invasion and migration by activating the TGF-β/Smad3 pathway in oral squamous cell carcinomas.Sci Rep. 2023 Sep 10;13(1):14922. doi: 10.1038/s41598-023-41504-8. Sci Rep. 2023. PMID: 37691034 Free PMC article.
-
Identification of HLA-A2-restricted CTL epitopes of a novel tumour-associated antigen, KIF20A, overexpressed in pancreatic cancer.Br J Cancer. 2011 Jan 18;104(2):300-7. doi: 10.1038/sj.bjc.6606052. Epub 2010 Dec 21. Br J Cancer. 2011. PMID: 21179034 Free PMC article.
-
Integrated genomic approaches identify upregulation of SCRN1 as a novel mechanism associated with acquired resistance to erlotinib in PC9 cells harboring oncogenic EGFR mutation.Oncotarget. 2016 Mar 22;7(12):13797-809. doi: 10.18632/oncotarget.7318. Oncotarget. 2016. PMID: 26883194 Free PMC article.
References
-
- Neugut AI, Hayek M, Howe G. Epidemiology of gastric cancer. Semin Oncol 1996; 23: 281–91. - PubMed
-
- Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev 2000; 26: 243–55. - PubMed
-
- Macdonald JS. Advances in the therapy of gastric cancer. Gastric Cancer 2002; 5 (Suppl. 1): 35–40. - PubMed
-
- Van der Bruggen P, Traversari C, Chomez P et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254: 1643–7. - PubMed
-
- Bjorkman PA, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatiblity antigen, HLA‐A2. Nature 1987; 329: 506–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

