Acute stress and nicotine cues interact to unveil locomotor arousal and activity-dependent gene expression in the prefrontal cortex
- PMID: 16631128
- PMCID: PMC1698504
- DOI: 10.1016/j.biopsych.2006.03.002
Acute stress and nicotine cues interact to unveil locomotor arousal and activity-dependent gene expression in the prefrontal cortex
Abstract
Background: This study examines the interactive effects of acute stress and nicotine-associated contextual cues on locomotor activity and activity-dependent gene expression in subregions of the prefrontal cortex.
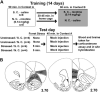
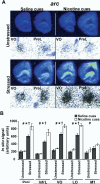
Methods: Locomotor activity of rats was measured in a context associated with either low-dose nicotine or saline administration with or without 5 minutes of pre-exposure to ferrets, a nonphysical stressor. After 45 minutes in the test environment, plasma corticosterone levels and mRNA levels of the immediate-early genes Arc, NGFI-B, and c-Fos in prefrontal and primary motor cortical subregions were measured.
Results: Stress alone increased plasma corticosterone and prefrontal cortex gene expression. Low-dose nicotine cues had no effect on corticosterone levels nor did they elicit conditioned motor activation, and they caused minor elevations in gene expression. Stress and low-dose nicotine cues, however, interacted to elicit conditioned motor activation and further increases in early response gene expression in prefrontal but not in the primary motor cortical subregions.
Conclusions: Stress interacts with nicotine-associated cues to uncover locomotor arousal, a state associated with prefrontal neuronal activation and immediate early gene expression. Thus, in nicotine-experienced individuals, stress may be an important determinant of subjective reactivity and prefrontal cortex activation that occurs in response to nicotine-associated cues.
Figures



References
-
- Adamec R. Does long term potentiation in periacqueductal gray (PAG) mediate lasting changes in rodent anxiety-like behavior (ALB) produced by predator stress?-Effects of low frequency stimulation (LFS) of PAG on place preference and changes in ALB produced by predator stress. Behav Brain Res. 2001;120:111–135. - PubMed
-
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: Dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–145. - PubMed
-
- Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: Assessment of the US-preexposure effect. Behav Brain Res. 2003;143:65–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

