A cortical potential imaging study from simultaneous extra- and intracranial electrical recordings by means of the finite element method
- PMID: 16631381
- PMCID: PMC1866308
- DOI: 10.1016/j.neuroimage.2006.02.027
A cortical potential imaging study from simultaneous extra- and intracranial electrical recordings by means of the finite element method
Abstract
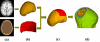
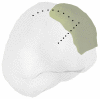
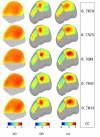
In the present study, we have validated the cortical potential imaging (CPI) technique for estimating cortical potentials from scalp EEG using simultaneously recorded electrocorticogram (ECoG) in the presence of strong local inhomogeneity, i.e., Silastic ECoG grid(s). The finite element method (FEM) was used to model the realistic postoperative head volume conductor, which includes the scalp, skull, cerebrospinal fluid (CSF) and brain, as well as the Silastic ECoG grid(s) implanted during the surgical evaluation in epilepsy patients, from the co-registered magnetic resonance (MR) and computer tomography (CT) images. A series of computer simulations were conducted to evaluate the present FEM-based CPI technique and to assess the effect of the Silastic ECoG grid on the scalp EEG forward solutions. The present simulation results show that the Silastic ECoG grid has substantial influence on the scalp potential forward solution due to the distortion of current pathways in the presence of the extremely low conductive materials. On the other hand, its influence on the estimated cortical potential distribution is much less than that on the scalp potential distribution. With appropriate numerical modeling and inverse estimation techniques, we have demonstrated the feasibility of estimating the cortical potentials from the scalp EEG with the implanted Silastic ECoG gird(s), in both computer simulations and in human experimentation. In an epilepsy patient undergoing surgical evaluation, the cortical potentials were reconstructed from the simultaneously recorded scalp EEG, in which main features of spatial patterns during interictal spike were preserved and over 0.75 correlation coefficient value was obtained between the recorded and estimated cortical potentials. The FEM-based CPI technique provides a means of connecting the simultaneous recorded ECoG and the scalp EEG and promises to become an effective tool to evaluate and validate CPI techniques using clinic data.
Figures








References
-
- Babiloni F, Babiloni C, Carducci F, Fattorini L, Anello C, Onorati P, Urbano A. High resolution EEG: A new model-dependent spatial deblurring method using a realisticallyshaped MR-constructed subject’s head model. Electroenceph. Clin. Neurophysiol. 1997;102:69–80. - PubMed
-
- Babiloni F, Babiloni C, Carducci F, Romani GL, Rossini PM, Angelone LM, Cincotti F. Multimodal integration of high-resolution EEG and functional magnetic resonance imaging data: a simulation study. Neuroimage. 2003;19:1–15. - PubMed
-
- Cohen D, Cuffin BN, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, Ives J, Kennedy JG, Schomer DL. MEG versus EEG localization test using implanted sources in the human brain. Ann Neurol. 1990;28:811–817. - PubMed
-
- Cuffin BN, Cohen D. Comparison of the magnetoencephalogram and electroencephalogram. Electroencephalogr. Clin. Neurophysiol. 1979;47:132–146. - PubMed
-
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J. Cognit. Neurosci. 1993;5:162–176. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

