A computational protocol for the integration of the monotopic protein prostaglandin H2 synthase into a phospholipid bilayer
- PMID: 16632499
- PMCID: PMC1483072
- DOI: 10.1529/biophysj.105.077784
A computational protocol for the integration of the monotopic protein prostaglandin H2 synthase into a phospholipid bilayer
Abstract
Prostaglandin H2 synthase (PGHS) synthesizes PGH2, a prostaglandin precursor, from arachidonic acid and was the first monotopic enzyme to have its structure experimentally determined. Both isozymes of PGHS are inhibited by nonsteroidal antiinflammatory drugs, an important class of drugs that are the primary means of relieving pain and inflammation. Selectively inhibiting the second isozyme, PGHS-2, minimizes the gastrointestinal side-effects. This had been achieved by the new PGHS-2 selective NSAIDs (i.e., COX-2 inhibitors) but it has been recently suggested that they suffer from additional side-effects. The design of these drugs only made use of static structures from x-ray crystallographic experiments. Investigating the dynamics of both PGHS-1 and PGHS-2 using classical molecular dynamics is expected to generate new insight into the differences in behavior between the isozymes, and therefore may allow improved PGHS-2 selective inhibitors to be designed. We describe a molecular dynamics protocol that integrates PGHS monomers into phospholipid bilayers, thereby producing in silico atomistic models of the PGHS system. Our protocol exploits the vacuum created beneath the protein when several lipids are removed from the top leaflet of the bilayer. The protein integrates into the bilayer during the first 5 ns in a repeatable process. The integrated PGHS monomer is stable and forms multiple hydrogen bonds between the phosphate groups of the lipids and conserved basic residues (Arg, Lys) on the protein. These interactions stabilize the system and are similar to interactions observed for transmembrane proteins.
Figures
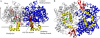









Similar articles
-
Anchoring of a monotopic membrane protein: the binding of prostaglandin H2 synthase-1 to the surface of a phospholipid bilayer.Eur Biophys J. 2000;29(6):439-54. doi: 10.1007/pl00006649. Eur Biophys J. 2000. PMID: 11081405
-
The analysis of nonsteroidal antiinflammatory drug selectivity in prostaglandin G/H synthase (PGHS)-null cells.Inflamm Res. 2001 Jun;50(6):327-32. doi: 10.1007/PL00000252. Inflamm Res. 2001. PMID: 11475335
-
PGH synthase isoenzyme selectivity: the potential for safer nonsteroidal antiinflammatory drugs.Am J Med. 1993 Aug 9;95(2A):40S-44S. doi: 10.1016/0002-9343(93)90396-7. Am J Med. 1993. PMID: 8357002 Review.
-
The membrane binding domains of prostaglandin endoperoxide H synthases 1 and 2. Peptide mapping and mutational analysis.J Biol Chem. 1999 Nov 12;274(46):32936-42. doi: 10.1074/jbc.274.46.32936. J Biol Chem. 1999. PMID: 10551860
-
Interactions of PGH synthase isozymes-1 and -2 with NSAIDs.Ann N Y Acad Sci. 1994 Nov 15;744:50-7. doi: 10.1111/j.1749-6632.1994.tb52723.x. Ann N Y Acad Sci. 1994. PMID: 7825862 Review.
Cited by
-
Phospholipid actions on PGHS-1 and -2 cyclooxygenase kinetics.Prostaglandins Other Lipid Mediat. 2008 Mar;85(3-4):134-43. doi: 10.1016/j.prostaglandins.2007.12.001. Epub 2007 Dec 8. Prostaglandins Other Lipid Mediat. 2008. PMID: 18201917 Free PMC article.
-
Capturing spontaneous partitioning of peripheral proteins using a biphasic membrane-mimetic model.J Phys Chem B. 2011 Jun 2;115(21):7029-37. doi: 10.1021/jp109631y. Epub 2011 May 11. J Phys Chem B. 2011. PMID: 21561114 Free PMC article.
-
Computational modeling of poly(alkylthiophene) conductive polymer insertion into phospholipid bilayers.Langmuir. 2007 Oct 9;23(21):10672-81. doi: 10.1021/la070214v. Epub 2007 Sep 15. Langmuir. 2007. PMID: 17867709 Free PMC article.
-
Characterization of Lipid-Protein Interactions and Lipid-Mediated Modulation of Membrane Protein Function through Molecular Simulation.Chem Rev. 2019 May 8;119(9):6086-6161. doi: 10.1021/acs.chemrev.8b00608. Epub 2019 Apr 12. Chem Rev. 2019. PMID: 30978005 Free PMC article. Review.
-
Alchembed: A Computational Method for Incorporating Multiple Proteins into Complex Lipid Geometries.J Chem Theory Comput. 2015 Jun 9;11(6):2743-2754. doi: 10.1021/ct501111d. Epub 2015 May 14. J Chem Theory Comput. 2015. PMID: 26089745 Free PMC article.
References
-
- Picot, D., P. Loll, and R. Garavito. 1994. The x-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 367:243–249. - PubMed
-
- Wendt, K. U., K. Poralla, and G. E. Schulz. 1997. Structure and function of a squalene cyclase. Science. 277:1811–1815. - PubMed
-
- Binda, C., P. Newton-Vinson, F. Hubálek, D. E. Edmondson, and A. Mattevi. 2002. Structure of human monoamine oxidase-b, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 9:22–26. - PubMed
-
- Bracey, M. H., M. A. Hanson, K. R. Masuda, R. C. Stevens, and B. F. Cravatt. 2002. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science. 298:1793–1796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

