Enzymatic pathways involved in the generation of endothelin-1(1-31) from exogenous big endothelin-1 in the rabbit aorta
- PMID: 16633356
- PMCID: PMC1751794
- DOI: 10.1038/sj.bjp.0706735
Enzymatic pathways involved in the generation of endothelin-1(1-31) from exogenous big endothelin-1 in the rabbit aorta
Abstract
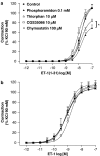
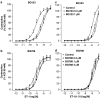
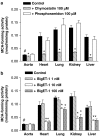
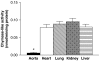
We investigated whether blood vessels contribute to the production of ET-1(1-31) from exogenous big endothelin-1 (BigET-1) in the rabbit and assessed which enzymes are involved in this process. Vascular reactivity experiments, using standard muscle bath procedures, showed that BigET-1 induces contraction in endothelium-intact rabbit aortic rings. Preincubation of the rings with phosphoramidon, CGS35066 or thiorphan reduced BigET-1-induced contraction. Conversely, chymostatin did not affect BigET-1-induced contraction. Thiorphan and phosphoramidon, but not CGS35066 or chymostatin, reduced ET-1(1-31)-induced contraction. None of the enzymatic inhibitors affected the contraction afforded by ET-1.BQ123-, but not BQ788-, selective antagonists for ET(A) and ET(B) receptors, respectively, produced concentration-dependent rightward displacements of the ET-1(1-31) and ET-1 concentration-response curves. By the use of enzymatic assays, we found that the aorta, as well as the heart, lung, kidney and liver, possess a chymase-like activity. Enzyme immunoassays detected significant levels of Ir-ET-1(1-31) in bathing medium of aortas after the addition of BigET-1 (30 nM). Neither thiorphan nor chymostatin altered the levels of Ir-ET-1(1-31). Conversely, the levels of Ir-ET-1(1-31) were increased in the presence of phosphoramidon. This marked increase of the 31-amino-acid peptide was abolished when phosphoramidon and chymostatin were added simultaneously. The major new finding of the present work is that the rabbit aorta generates ET-1(1-31) from exogenously administered BigET-1. Additionally, by measuring the production of ET-1(1-31), we showed that a chymase-like enzyme is involved in this process when ECE and NEP are inhibited by phosphoramidon. Our results also suggest that ET-1(1-31) is an alternate intermediate in the production of ET-1 following BigET-1 administration. Finally, we showed that NEP is the predominant enzymatic pathway involved in the cleavage of ET-1(1-31) to a bioactive metabolite that will act on ET(A) receptors to induce contraction in the rabbit aorta.
Figures



References
-
- AKASU M., URATA H., KINOSHITA A., SASAGURI M., IDEISHI M., ARAKAWA K. Differences in tissue angiotensin II-forming pathways by species and organs in vitro. Hypertension. 1998;32:514–520. - PubMed
-
- BROSSEAU J.P., D'ORLEANS-JUSTE P., NEUGEBAUER W.A. Development of an efficient strategy for the synthesis of the ETB receptor antagonist BQ-788 and some related analogues. Peptides. 2005;26:1441–1453. - PubMed
-
- CRAIG S.S., SCHWARTZ L.B. Tryptase and chymase, markers of distinct types of human mast cells. Immunol. Res. 1989;8:130–148. - PubMed
-
- DE CAMPO B.A., GOLDIE R.G., JENG A.Y., HENRY P.J. Role of endothelin-converting enzyme, chymase and neutral endopeptidase in the processing of bigET-1, ET-1(1-21) and ET-1(1–31) in the trachea of allergic mice. Clinical Sciences. 2002;103 (Suppl 48):353S–356S. - PubMed
-
- D'ORLEANS-JUSTE P., LIDBURY P.S., WARNER T.D., VANE J.R. Intravascular big endothelin increases circulating levels of endothelin-1 and prostanoids in the rabbit. Biochem. Pharmacol. 1990;39:R21–R22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

