Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury
- PMID: 16634077
- PMCID: PMC3951737
- DOI: 10.1002/hipo.20183
Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury
Abstract
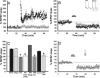
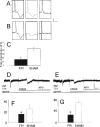
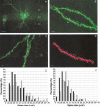
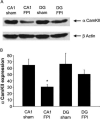
Traumatic brain injury (TBI) is a significant health issue that often causes enduring cognitive deficits, in particular memory dysfunction. The hippocampus, a structure crucial in learning and memory, is frequently damaged during TBI. Since long-term potentiation (LTP) is the leading cellular model underlying learning and memory, this study was undertaken to examine how injury affects area CA1 LTP in mice using lateral fluid percussion injury (FPI). Brain slices derived from FPI animals demonstrated an inability to induce LTP in area CA1 7 days postinjury. However, area CA1 long-term depression could be induced in neurons 7 days postinjury, demonstrating that some forms of synaptic plasticity can still be elicited. Using a multi-disciplined approach, potential mechanisms underlying the inability to induce and maintain area CA1 LTP were investigated. This study demonstrates that injury leads to significantly smaller N-methyl-D-aspartate potentials and glutamate-induced excitatory currents, increased dendritic spine size, and decreased expression of alpha-calcium calmodulin kinase II. These findings may underlie the injury-induced lack of LTP and thus, contribute to cognitive impairments often associated with TBI. Furthermore, these results provide attractive sites for potential therapeutic intervention directed toward alleviating the devastating consequences of human TBI.
Figures
Similar articles
-
Decreased calcium/calmodulin-dependent protein kinase II and protein kinase C activities mediate impairment of hippocampal long-term potentiation in the olfactory bulbectomized mice.J Neurochem. 2006 Apr;97(1):22-9. doi: 10.1111/j.1471-4159.2006.03710.x. Epub 2006 Mar 3. J Neurochem. 2006. PMID: 16515554
-
Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition.Cell. 2005 Oct 7;123(1):105-18. doi: 10.1016/j.cell.2005.07.033. Cell. 2005. PMID: 16213216
-
Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus.Eur J Neurosci. 2005 Jul;22(1):169-78. doi: 10.1111/j.1460-9568.2005.04205.x. Eur J Neurosci. 2005. PMID: 16029206
-
Expression mechanisms underlying NMDA receptor-dependent long-term potentiation.Ann N Y Acad Sci. 1999 Apr 30;868:515-25. doi: 10.1111/j.1749-6632.1999.tb11320.x. Ann N Y Acad Sci. 1999. PMID: 10414328 Review.
-
Long-term potentiation in cultured hippocampal neurons.Semin Cell Dev Biol. 2011 Jul;22(5):506-13. doi: 10.1016/j.semcdb.2011.07.017. Epub 2011 Jul 22. Semin Cell Dev Biol. 2011. PMID: 21807105 Review.
Cited by
-
Disrupted Hippocampal Theta-Gamma Coupling and Spike-Field Coherence Following Experimental Traumatic Brain Injury.bioRxiv [Preprint]. 2024 Sep 12:2024.05.30.596704. doi: 10.1101/2024.05.30.596704. bioRxiv. 2024. PMID: 39314320 Free PMC article. Preprint.
-
Making Waves in the Brain: What Are Oscillations, and Why Modulating Them Makes Sense for Brain Injury.Front Syst Neurosci. 2016 Apr 7;10:30. doi: 10.3389/fnsys.2016.00030. eCollection 2016. Front Syst Neurosci. 2016. PMID: 27092062 Free PMC article. Review.
-
MMP-9 Contributes to Dendritic Spine Remodeling Following Traumatic Brain Injury.Neural Plast. 2019 May 6;2019:3259295. doi: 10.1155/2019/3259295. eCollection 2019. Neural Plast. 2019. PMID: 31198417 Free PMC article.
-
Blocking leukotriene synthesis attenuates the pathophysiology of traumatic brain injury and associated cognitive deficits.Exp Neurol. 2014 Jun;256:7-16. doi: 10.1016/j.expneurol.2014.03.008. Epub 2014 Mar 25. Exp Neurol. 2014. PMID: 24681156 Free PMC article.
-
Mild Blast Injury Produces Acute Changes in Basal Intracellular Calcium Levels and Activity Patterns in Mouse Hippocampal Neurons.J Neurotrauma. 2018 Jul 1;35(13):1523-1536. doi: 10.1089/neu.2017.5029. Epub 2018 Apr 10. J Neurotrauma. 2018. PMID: 29343209 Free PMC article.
References
-
- Barth JT, Macciocchi SN, Giordani B, Rimel R, Jane JA, Boll TJ. Neuropsychological sequelae of minor head injury. Neuro-surgery. 1983;13:529–533. - PubMed
-
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. - PubMed
-
- Bennett-Levy JM. Long-term effects of severe closed head injury on memory: Evidence from a consecutive series of young adults. Acta Neurol Scand. 1984;70:285–298. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

