Synthesis and thermodynamic evaluation of mixed hexadentate linear iron chelators containing hydroxypyridinone and terephthalamide units
- PMID: 16634594
- PMCID: PMC3685440
- DOI: 10.1021/ic052111a
Synthesis and thermodynamic evaluation of mixed hexadentate linear iron chelators containing hydroxypyridinone and terephthalamide units
Abstract
A series of new linear iron chelators containing hydroxypyridinone and terephthalamide (TAMmeg) moieties have been prepared. All are hexadentate ligands composed of a systematically varied combination of methyl-3,2-hydroxypyridinone and 2,3-dihydroxyterephthalamide binding units; most are based on a spermidine scaffold, but one incorporates the bifunctional 2,3-dihydroxyterephthalamide unit as an integral part of the backbone. Protonation and ferric iron complex formation constants have been determined from solution thermodynamic studies, giving log beta(110) values of 25.7, 30.7, 36.3, 43.8, and 45.0, respectively. The ferric complexes display reversible reduction potentials from -276 to -1032 mV (measured relative to the normal hydrogen electrode) in alkaline solution. The incremental replacement of hydroxypyridinone units by terephthalamide binding groups progressively reduces the ligand acidity, markedly increases the iron-chelate stability, and improves the selectivity for the ferric ion over the ferrous ion. While the majority of iron chelators forming very stable ferric complexes are based on a tripodal backbone such as TREN, the ferric 5-LIO(TAMmeg)(2)(TAM) complex, despite its nontripodal scaffold, is one of the most stable iron complexes yet reported. Moreover, the high affinity for the ferric ion of the discussed linear ligands strongly correlates with their ability to remove iron in vivo.
Figures






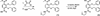
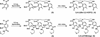


References
-
- Jurchen KMC, Raymond KN. Ferric Ion Sequestering Agents. 42. Part 41. Inorg. Chem. 2006 In Press.
-
- Crichton RR. Inorganic Biochemistry of Iron Metabolism. New York, London: Ellis Hardwood; 1991.
-
- Koppenol WH. In: Iron Chelators: New Development Strategies. Badman DG, Bergeron RJ, Brittenham GM, editors. Ponte Vedra Beach, FL: The Saratoga Group; 2000. pp. 3–10.
-
- Hershko C. Reviews in Clinical and Experimental Hematology. 2000;4:337–361.
-
- Hershko C, Konijn AM, Link G. In: Iron Chelators: New Development Strategies. Badman DG, Bergeron RJ, Brittenham GM, editors. Ponte Vedra Beach, FL: The Saratoga Group; 2000. pp. 157–176.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

