Circulating IgA from acute stage of childhood Henoch-Schönlein purpura can enhance endothelial interleukin (IL)-8 production through MEK/ERK signalling pathway
- PMID: 16634798
- PMCID: PMC1809646
- DOI: 10.1111/j.1365-2249.2006.03076.x
Circulating IgA from acute stage of childhood Henoch-Schönlein purpura can enhance endothelial interleukin (IL)-8 production through MEK/ERK signalling pathway
Abstract
Recently, sera from children with active Henoch-Schönlein purpura (HSP) have been found to enhance interleukin (IL)-8 production by human umbilical venous endothelial cells (HUVEC). To further determine the possible factor with the ability to enhance endothelial IL-8 production in sera from acute stage of HSP, 10 children with HSP at the acute stage and 10 healthy controls were enrolled. IgA antiendothelial cell antibodies (AECA) were detected by cell-based ELISA. Active sera with or without pretreatment with anti-human IgA antibody, sera of controls, and immunoglobulin A (IgA) derived from sera were used to stimulate the HUVEC. The ability of these factors to enhance endothelial IL-8 production was evaluated. Furthermore, signalling pathways were also assayed by different inhibitors, and confirmed by immunoblotting. Serum levels of IgA AECA in HPS patients at the acute stage were significantly higher than in controls (P < 0.001). The active sera could enhance endothelial IL-8 production (P = 0.004, compared with control sera), and the ability of these sera was mostly abolished when pretreated with fixed anti-human IgA antibody. The supernatant IL-8 levels of endothelial cells stimulated by IgA derived from acute stage of HSP were statistically higher than controls (P < 0.001). PD98059, an inhibitor of ERK phosphorylation, significantly reduced IgA AECA-stimulated endothelial IL-8. IgA AECA also enhanced the phosphorylation of ERK1 with a time-dependent manner. Together with these findings, it is concluded that IgA AECA derived from acute stage of HSP may bind to endothelial and enhance endothelial cells to produce IL-8 via MEK/REK signalling pathway.
Figures



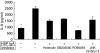

References
-
- Yang YH, Hung CF, Hsu CR, et al. A nationwide survey on epidemiological characteristics of childhood Henoch-Schönlein Purpura in Taiwan. Rheumatology. 2005;44:618–22. - PubMed
-
- Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002;360:1197–202. - PubMed
-
- Ballinger S. Henoch-Schonlein purpura. Curr Opin Rheumatol. 2003;15:591–4. - PubMed
-
- Bissonnette R, Dansereau A, D'Amico P, Pateneaude JV, Paradis J. Perforation of large and small bowel in Henoch-Schonlein purpura. Int J Dermatol. 1997;36:361–3. - PubMed
-
- Coppo R, Mazzucco G, Cagnoli L, Lupo A, Schena FP. Long-term prognosis of Henoch-Schonlein nephritis in adults and children. Italian Group of Renal Immunopathology Collaborative Study on Henoch-Schonlein purpura. Nephrol Dial Transplant. 1997;12:2277–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

