NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis
- PMID: 16636131
- PMCID: PMC2121204
- DOI: 10.1084/jem.20051911
NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis
Abstract
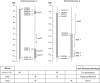
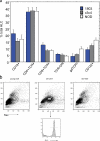
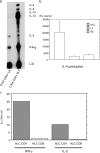
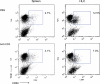
Primary biliary cirrhosis (PBC) is an autoimmune disease with a strong genetic component characterized by biliary ductular inflammation with eventual liver cirrhosis. The serologic hallmark of PBC is antimitochondrial antibodies that react with the pyruvate dehydrogenase complex, targeting the inner lipoyl domain of the E2 subunit (anti-PDC-E2). Herein we demonstrate that NOD.c3c4 mice congenically derived from the nonobese diabetic strain develop an autoimmune biliary disease (ABD) that models human PBC. NOD.c3c4 (at 9-10 wk, before significant biliary pathology) develop antibodies to PDC-E2 that are specific for the inner lipoyl domain. Affected areas of biliary epithelium are infiltrated with CD3+, CD4+, and CD8+ T cells, and treatment of NOD.c3c4 mice with monoclonal antibody to CD3 protects from ABD. Furthermore, NOD.c3c4-scid mice develop disease after adoptive transfer of splenocytes or CD4+ T cells, demonstrating a central role for T cells in pathogenesis. Histological analysis reveals destructive cholangitis, granuloma formation, and eosinophilic infiltration as seen in PBC, although, unlike PBC, the extrahepatic biliary ducts are also affected. Using a congenic mapping approach, we define the first ABD (Abd) locus, Abd1. These results identify the NOD.c3c4 mouse as the first spontaneous mouse model of PBC.
Figures




Comment in
-
An autoimmune biliary disease mouse model for primary biliary cirrhosis: something for everyone.Hepatology. 2006 Oct;44(4):1047-50. doi: 10.1002/hep.21390. Hepatology. 2006. PMID: 17006941 No abstract available.
References
-
- Gershwin, M.E., A.A. Ansari, I.R. Mackay, Y. Nakanuma, A. Nishio, M.J. Rowley, and R.L. Coppel. 2000. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol. Rev. 174:210–225. - PubMed
-
- Gershwin, M.E., I.R. Mackay, A. Sturgess, and R.L. Coppel. 1987. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J. Immunol. 138:3525–3531. - PubMed
-
- Van de Water, J., A.A. Ansari, C.D. Surh, R. Coppel, T. Roche, H. Bonkovsky, M. Kaplan, and M.E. Gershwin. 1991. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T cell response of primary biliary cirrhosis. J. Immunol. 146:89–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

