A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia
- PMID: 16636133
- PMCID: PMC2121202
- DOI: 10.1084/jem.20052398
A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia
Erratum in
-
A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia.J Exp Med. 2015 May 4;212(5):825. doi: 10.1084/jem.2005239804172015c. J Exp Med. 2015. PMID: 25941322 Free PMC article. No abstract available.
Abstract
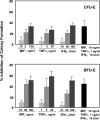
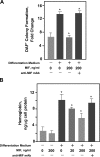
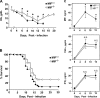
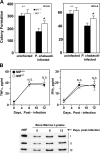
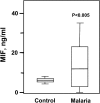
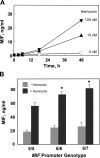
The pathogenesis of malarial anemia is multifactorial, and the mechanisms responsible for its high mortality are poorly understood. Studies indicate that host mediators produced during malaria infection may suppress erythroid progenitor development (Miller, K.L., J.C. Schooley, K.L. Smith, B. Kullgren, L.J. Mahlmann, and P.H. Silverman. 1989. Exp. Hematol. 17:379-385; Yap, G.S., and M.M. Stevenson. 1991. Ann. NY Acad. Sci. 628:279-281). We describe an intrinsic role for macrophage migration inhibitory factor (MIF) in the development of the anemic complications and bone marrow suppression that are associated with malaria infection. At concentrations found in the circulation of malaria-infected patients, MIF suppressed erythropoietin-dependent erythroid colony formation. MIF synergized with tumor necrosis factor and gamma interferon, which are known antagonists of hematopoiesis, even when these cytokines were present in subinhibitory concentrations. MIF inhibited erythroid differentiation and hemoglobin production, and it antagonized the pattern of mitogen-activated protein kinase phosphorylation that normally occurs during erythroid progenitor differentiation. Infection of MIF knockout mice with Plasmodium chabaudi resulted in less severe anemia, improved erythroid progenitor development, and increased survival compared with wild-type controls. We also found that human mononuclear cells carrying highly expressed MIF alleles produced more MIF when stimulated with the malarial product hemozoin compared with cells carrying low expression MIF alleles. These data suggest that polymorphisms at the MIF locus may influence the levels of MIF produced in the innate response to malaria infection and the likelihood of anemic complications.
Figures


References
-
- Miller, L., D. Baruch, K. Marsh, and O. Doumbo. 2002. The pathogenic basis of malaria. Nature. 415:673–679. - PubMed
-
- Breman, J.G. 2001. The ears of the hippopotamus: manifestations, determinants, and the estimate of the malaria burden. Am. J. Trop. Med. Hyg. 64:S1–11. - PubMed
-
- Nagel, R. 2002. Malarial anemia. Hemoglobin. 26:329–343. - PubMed
-
- McDevitt, M., J. Xie, V. Gordeuk, and R. Bucala. 2004. The anemia of malaria infection: role of inflammatory cytokines. Curr. Hematol. Rep. 3:97–106. - PubMed
-
- Chang, K.-H., and M.M. Stevenson. 2004. Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int. J. Parasitol. 34:1501–1516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

