Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates
- PMID: 16636134
- PMCID: PMC2121207
- DOI: 10.1084/jem.20052433
Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates
Abstract
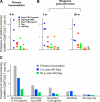
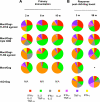
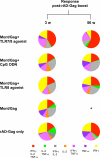
There is a remarkable heterogeneity in the functional profile (quality) of T cell responses. Importantly, the magnitude and/or quality of a response required for protection may be different depending on the infection. Here, we assessed the capacity of different Toll like receptor (TLR)-binding compounds to influence T helper cell (Th)1 and CD8+ T cell responses when used as adjuvants in nonhuman primates (NHP) with HIV Gag as a model antigen. NHP were immunized with HIV Gag protein emulsified in Montanide ISA 51, an oil-based adjuvant, with or without a TLR7/8 agonist, a TLR8 agonist, or the TLR9 ligand cytosine phosphate guanosine oligodeoxynucleotides (CpG ODN), and boosted 12 wk later with a replication-defective adenovirus-expressing HIV-Gag (rAD-Gag). Animals vaccinated with HIV Gag protein/Montanide and CpG ODN or the TLR7/8 agonist had higher frequencies of Th1 responses after primary immunization compared to all other vaccine groups. Although the rAD-Gag boost did not elevate the frequency of Th1 memory cytokine responses, there was a striking increase in HIV Gag-specific CD8+ T cell responses after the boost in all animals that had received a primary immunization with any of the TLR adjuvants. Importantly, the presence and type of TLR adjuvant used during primary immunization conferred stability and dramatically influenced the magnitude and quality of the Th1 and CD8+ T cell responses after the rAD-Gag boost. These data provide insights for designing prime-boost immunization regimens to optimize Th1 and CD8+ T cell responses.
Figures




References
-
- Seder, R.A., and A.V. Hill. 2000. Vaccines against intracellular infections requiring cellular immunity. Nature. 406:793–798. - PubMed
-
- Baba, T.W., Y.S. Jeong, D. Pennick, R. Bronson, M.F. Greene, and R.M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 267:1820–1825. - PubMed
-
- Shiver, J.W., T.M. Fu, L. Chen, D.R. Casimiro, M.E. Davies, R.K. Evans, Z.Q. Zhang, A.J. Simon, W.L. Trigona, S.A. Dubey, et al. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 415:331–335. - PubMed
-
- Seaman, M.S., L. Xu, K. Beaudry, K.L. Martin, M.H. Beddall, A. Miura, A. Sambor, B.K. Chakrabarti, Y. Huang, R. Bailer, et al. 2005. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J. Virol. 79:2956–2963. - PMC - PubMed
-
- Donnelly, J.J., B. Wahren, and M.A. Liu. 2005. DNA vaccines: progress and challenges. J. Immunol. 175:633–639. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

