Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy
- PMID: 16636135
- PMCID: PMC3212727
- DOI: 10.1084/jem.20052494
Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy
Abstract
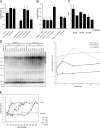
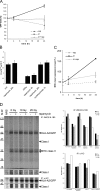
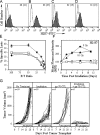
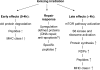
Radiotherapy is one of the most successful cancer therapies. Here the effect of irradiation on antigen presentation by MHC class I molecules was studied. Cell surface expression of MHC class I molecules was increased for many days in a radiation dose-dependent manner as a consequence of three responses. Initially, enhanced degradation of existing proteins occurred which resulted in an increased intracellular peptide pool. Subsequently, enhanced translation due to activation of the mammalian target of rapamycin pathway resulted in increased peptide production, antigen presentation, as well as cytotoxic T lymphocyte recognition of irradiated cells. In addition, novel proteins were made in response to gamma-irradiation, resulting in new peptides presented by MHC class I molecules, which were recognized by cytotoxic T cells. We show that immunotherapy is successful in eradicating a murine colon adenocarcinoma only when preceded by radiotherapy of the tumor tissue. Our findings indicate that directed radiotherapy can improve the efficacy of tumor immunotherapy.
Figures


References
-
- Reits, E.A., J.C. Vos, M. Gromme, and J. Neefjes. 2000. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 404:774–778. - PubMed
-
- Schubert, U., L.C. Anton, J. Gibbs, C.C. Norbury, J.W. Yewdell, and J.R. Bennink. 2000. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 404:770–774. - PubMed
-
- Reits, E., J. Neijssen, C. Herberts, W. Benckhuijsen, L. Janssen, J.W. Drijfhout, and J. Neefjes. 2004. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity. 20:495–506. - PubMed
-
- Reits, E., A. Griekspoor, J. Neijssen, T. Groothuis, K. Jalink, P. van Veelen, H. Janssen, J. Calafat, J.W. Drijfhout, and J. Neefjes. 2003. Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity. 18:97–108. - PubMed
-
- Yewdell, J.W. 2001. Not such a dismal science: the economics of protein synthesis, folding, degradation and antigen processing. Trends Cell Biol. 11:294–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

