Lysosomal biogenesis and function is critical for necrotic cell death in Caenorhabditis elegans
- PMID: 16636145
- PMCID: PMC2063814
- DOI: 10.1083/jcb.200511103
Lysosomal biogenesis and function is critical for necrotic cell death in Caenorhabditis elegans
Abstract
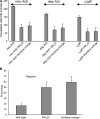
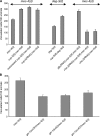
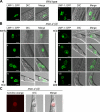
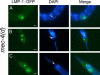
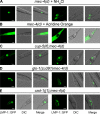
Necrotic cell death is defined by distinctive morphological characteristics that are displayed by dying cells (Walker, N.I., B.V. Harmon, G.C. Gobe, and J.F. Kerr. 1988. Methods Achiev. Exp. Pathol. 13:18-54). The cellular events that transpire during necrosis to generate these necrotic traits are poorly understood. Recent studies in the nematode Caenorhabditis elegans show that cytoplasmic acidification develops during necrosis and is required for cell death (Syntichaki, P., C. Samara, and N. Tavernarakis. 2005. Curr. Biol. 15:1249-1254). However, the origin of cytoplasmic acidification remains elusive. We show that the alkalization of endosomal and lysosomal compartments ameliorates necrotic cell death triggered by diverse stimuli. In addition, mutations in genes that result in altered lysosomal biogenesis and function markedly affect neuronal necrosis. We used a genetically encoded fluorescent marker to follow lysosome fate during neurodegeneration in vivo. Strikingly, we found that lysosomes fuse and localize exclusively around a swollen nucleus. In the advanced stages of cell death, the nucleus condenses and migrates toward the periphery of the cell, whereas green fluorescent protein-labeled lysosomal membranes fade, indicating lysosomal rupture. Our findings demonstrate a prominent role for lysosomes in cellular destruction during necrotic cell death, which is likely conserved in metazoans.
Figures
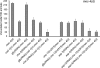
References
-
- Artal-Sanz, M., and N. Tavernarakis. 2005. Proteolytic mechanisms in necrotic cell death and neurodegeneration. FEBS Lett. 579:3287–3296. - PubMed
-
- Bakker, A.C., P. Webster, W.A. Jacob, and N.W. Andrews. 1997. Homotypic fusion between aggregated lysosomes triggered by elevated [Ca2+]i in fibroblasts. J. Cell Sci. 110:2227–2238. - PubMed

