Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning
- PMID: 16636147
- PMCID: PMC2063818
- DOI: 10.1083/jcb.200507119
Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning
Abstract
Loss of tuberin, the product of TSC2 gene, increases mammalian target of rapamycin (mTOR) signaling, promoting cell growth and tumor development. However, in cells expressing tuberin, it is not known how repression of mTOR signaling is relieved to activate this pathway in response to growth factors and how hamartin participates in this process. We show that hamartin colocalizes with hypophosphorylated tuberin at the membrane, where tuberin exerts its GTPase-activating protein (GAP) activity to repress Rheb signaling. In response to growth signals, tuberin is phosphorylated by AKT and translocates to the cytosol, relieving Rheb repression. Phosphorylation of tuberin at serines 939 and 981 does not alter its intrinsic GAP activity toward Rheb but partitions tuberin to the cytosol, where it is bound by 14-3-3 proteins. Thus, tuberin bound by 14-3-3 in response to AKT phosphorylation is sequestered away from its membrane-bound activation partner (hamartin) and its target GTPase (Rheb) to relieve the growth inhibitory effects of this tumor suppressor.
Figures





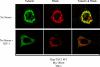


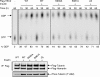

References
-
- Alessi, D.R., F.B. Caudwell, M. Andjelkovic, B.A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333–338. - PubMed
-
- Bjornsti, M.A., and P.J. Houghton. 2004. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer. 4:335–348. - PubMed
-
- Cheadle, J.P., M.P. Reeve, J.R. Sampson, and D.J. Kwiatkowski. 2000. Molecular genetic advances in tuberous sclerosis. Hum. Genet. 107:97–114. - PubMed
-
- Clark, G.J., M.S. Kinch, K. Rogers-Graham, S.M. Sebti, A.D. Hamilton, and C.J. Der. 1997. The Ras-related protein Rheb is farnesylated and antagonizes Ras signaling and transformation. J. Biol. Chem. 272:10608–10615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

