Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia
- PMID: 16636206
- PMCID: PMC2151517
- DOI: 10.1085/jgp.200509468
Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia
Abstract
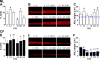
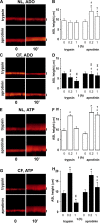
A key aspect of the lung's innate defense system is the ability of the superficial epithelium to regulate airway surface liquid (ASL) volume to maintain a 7-mum periciliary liquid layer (PCL), which is required for cilia to beat and produce mucus flow. The mechanisms whereby airway epithelia regulate ASL height to >or=7 microm are poorly understood. Using bumetanide as an inhibitor of Cl- secretion, and nystatin as an activator of Na+ absorption, we found that a coordinated "blending" of both Cl- secretion and Na+ absorption must occur to effect ASL volume homeostasis. We then investigated how ASL volume status is regulated by the underlying epithelia. Cilia were not critical to this process as (a) ASL volume was normal in cultures from patients with primary ciliary dyskinesia with immotile cilia, and (b) in normal cultures that had not yet undergone ciliogenesis. However, we found that maneuvers that mimic deposition of excess ASL onto the proximal airways, which occurs during mucociliary clearance and after glandular secretion, acutely stimulated Na+ absorption, suggesting that volume regulation was sensitive to changes in concentrations of soluble mediators in the ASL rather than alterations in ciliary beating. To investigate this hypothesis further, we added potential "soluble mediators" to the ASL. ASL volume regulation was sensitive to a channel-activating protein (CAP; trypsin) and a CAP inhibitor (aprotinin), which regulated Na+ absorption via changes in epithelial Na+ channel (ENaC) activity in both normal and cystic fibrosis cultures. ATP was also found to acutely regulate ASL volume by inducing secretion in normal and cystic fibrosis (CF) cultures, while its metabolite adenosine (ADO) evoked secretion in normal cultures but stimulated absorption in CF cultures. Interestingly, the amount of ASL/Cl- secretion elicited by ATP/ADO was influenced by the level of CAP-induced Na+ absorption, suggesting that there are important interactions between the soluble regulators which finely tune ASL volume.
Figures
References
-
- Alton, E.W., A. Khagani, M.H. Yacoub, and D.M. Geddes. 1989. Lack of effect of lung denervation on the measurement of potential difference after single-lung transplantation. N. Engl. J. Med. 320:1755 (letter). - PubMed
-
- Boucher, R.C. 1994. Human airway ion transport (Part 1). Am. J. Respir. Crit. Care Med. 150:271–281. - PubMed
-
- Bridges, R.J., B.B. Newton, J.M. Pilewski, D.C. Devor, C.T. Poll, and R.L. Hall. 2001. Na+ transport in normal and CF human bronchial epithelial cells is inhibited by BAY 39-9437. Am. J. Physiol. 281:L16–L23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

