NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions
- PMID: 16636276
- PMCID: PMC1459027
- DOI: 10.1073/pnas.0602006103
NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions
Abstract
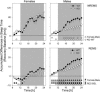
Because the transcription factor neuronal Per-Arnt-Sim-type signal-sensor protein-domain protein 2 (NPAS2) acts both as a sensor and an effector of intracellular energy balance, and because sleep is thought to correct an energy imbalance incurred during waking, we examined NPAS2's role in sleep homeostasis using npas2 knockout (npas2-/-) mice. We found that, under conditions of increased sleep need, i.e., at the end of the active period or after sleep deprivation (SD), NPAS2 allows for sleep to occur at times when mice are normally awake. Lack of npas2 affected electroencephalogram activity of thalamocortical origin; during non-rapid eye movement sleep (NREMS), activity in the spindle range (10-15 Hz) was reduced, and within the delta range (1-4 Hz), activity shifted toward faster frequencies. In addition, the increase in the cortical expression of the NPAS2 target gene period2 (per2) after SD was attenuated in npas2-/- mice. This implies that NPAS2 importantly contributes to the previously documented wake-dependent increase in cortical per2 expression. The data also revealed numerous sex differences in sleep; in females, sleep need accumulated at a slower rate, and REMS loss was not recovered after SD. In contrast, the rebound in NREMS time after SD was compromised only in npas2-/- males. We conclude that NPAS2 plays a role in sleep homeostasis, most likely at the level of the thalamus and cortex, where NPAS2 is abundantly expressed.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures





References
-
- Gu Y. Z., Hogenesch J. B., Bradfield C. A. Annu. Rev. Pharmacol. Toxicol. 2000;40:519–561. - PubMed
-
- Rutter J., Reick M., Wu L. C., McKnight S. L. Science. 2001;293:510–514. - PubMed
-
- Borbely A. A. Hum. Neurobiol. 1982;1:195–204. - PubMed
-
- Benington J. H., Heller H. C. Prog. Neurobiol. 1995;45:347–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

