Retinoic acid exerts dual regulatory actions on the expression and nuclear localization of interferon regulatory factor-1
- PMID: 16636311
- PMCID: PMC3843134
- DOI: 10.1177/153537020623100517
Retinoic acid exerts dual regulatory actions on the expression and nuclear localization of interferon regulatory factor-1
Abstract
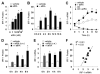
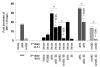
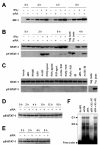
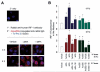
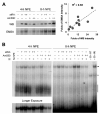
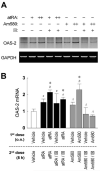
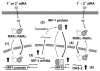
Interferon regulatory factor-1 (IRF-1), a transcription factor and tumor suppressor involved in cell growth regulation and immune responses, has been shown to be induced by all-trans retinoic acid (ATRA). However, the factors controlling the cellular location and activity of IRF-1 are not well understood. In this study, we examined the expression of IRF-1 and its nuclear localization, DNA-binding activity, and target gene expression in human mammary epithelial MCF10A cells, a model of breast epithelial cell differentiation and carcinogenesis. Following initial treatment with ATRA, IRF-1 mRNA and protein were induced within 2 hrs, reached a peak (>30-fold induction) at 8 hrs, and declined afterwards. IRF-1 protein was predominantly cytoplasmic during this treatment. Although a second dose of ATRA or Am580 (a related retinoid selective for retinoic acid receptor-alpha [RARalpha]), given 16 hrs after the first dose, restimulated IRF-1 mRNA and protein levels to a similar level to that obtained by the first dose, IRF-1 was predominantly concentrated in the nucleus after restimulation. ATRA and Am580 also increased nuclear RARalpha, whereas retinoid X receptor-alpha (RXRalpha)--a dimerization partner for RARalpha, was localized to the nucleus upon second exposure to ATRA. However, ATRA and Am580 did not regulate the expression or activation of signal transducer and activator of transcription-1 (STAT-1), a transcription factor capable of inducing the expression of IRF-1, indicating an STAT-1-independent mechanism of regulation by ATRA and Am580. The increase in nuclear IRF-1 after retinoid restimulation was accompanied by enhanced binding to an IRF-E DNA response element, and elevated expression of an IRF-1 target gene, 2',5'-oligoadenylate synthetase-2. The dual effect of retinoids in increasing IRF-1 mRNA and protein and in augmenting the nuclear localization of IRF-1 protein may be essential for maximizing the tumor suppressor activity and the immunosurveillance functions of IRF-1 in breast epithelial cells.
Figures

Similar articles
-
Physiological and receptor-selective retinoids modulate interferon gamma signaling by increasing the expression, nuclear localization, and functional activity of interferon regulatory factor-1.J Biol Chem. 2005 Oct 28;280(43):36228-36. doi: 10.1074/jbc.M505749200. Epub 2005 Aug 5. J Biol Chem. 2005. PMID: 16085646 Free PMC article.
-
Retinoic acid activates interferon regulatory factor-1 gene expression in myeloid cells.Blood. 1996 Jul 1;88(1):114-23. Blood. 1996. PMID: 8704165
-
Ubiquitinated or sumoylated retinoic acid receptor alpha determines its characteristic and interacting model with retinoid X receptor alpha in gastric and breast cancer cells.J Mol Endocrinol. 2004 Jun;32(3):595-613. doi: 10.1677/jme.0.0320595. J Mol Endocrinol. 2004. PMID: 15171703
-
Regulation of IRF and STAT gene expression by retinoic acid.Leuk Lymphoma. 1998 Jun;30(1-2):63-71. doi: 10.3109/10428199809050930. Leuk Lymphoma. 1998. PMID: 9669677 Review.
-
Retinoic acid homeostasis and disease.Curr Top Dev Biol. 2025;161:201-233. doi: 10.1016/bs.ctdb.2024.11.001. Epub 2024 Dec 6. Curr Top Dev Biol. 2025. PMID: 39870434 Review.
Cited by
-
Translational Potential of Therapeutics Targeting Regulatory Myeloid Cells in Tuberculosis.Front Cell Infect Microbiol. 2018 Sep 21;8:332. doi: 10.3389/fcimb.2018.00332. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30298121 Free PMC article. Review.
-
RIG-I is required for the inhibition of measles virus by retinoids.PLoS One. 2011;6(7):e22323. doi: 10.1371/journal.pone.0022323. Epub 2011 Jul 19. PLoS One. 2011. PMID: 21811588 Free PMC article.
-
Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines.BMC Cancer. 2007 Feb 23;7:34. doi: 10.1186/1471-2407-7-34. BMC Cancer. 2007. PMID: 17319941 Free PMC article.
-
Ameliorative effects of vitamins-loaded flavoured nanophytosomes fortified with star anise volatile oil against CsA-Induced liver and kidney injury in rats: Application in functional ice cream.Heliyon. 2023 Dec 19;10(1):e23894. doi: 10.1016/j.heliyon.2023.e23894. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38226243 Free PMC article.
-
CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade.Cancer Discov. 2018 Sep;8(9):1156-1175. doi: 10.1158/2159-8290.CD-17-1033. Epub 2018 Jul 16. Cancer Discov. 2018. PMID: 30012853 Free PMC article.
References
-
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. - PubMed
-
- Ross AC, Stephensen CB. Vitamin A and retinoids in antiviral responses. FASEB J. 1996;10:979–985. - PubMed
-
- Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. - PubMed
-
- Wei LN. Retinoids and receptor interacting protein 140 (RIP140) in gene regulation. Curr Med Chem. 2004;11:1527–1532. - PubMed
-
- Privalsky ML. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu Rev Physiol. 2004;66:315–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

