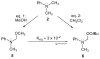
The oxidative mannich reaction catalyzed by dirhodium caprolactamate
- PMID: 16637627
- PMCID: PMC2610808
- DOI: 10.1021/ja061146m
The oxidative mannich reaction catalyzed by dirhodium caprolactamate
Abstract
Dirhodium caprolactamate [Rh2(cap)4] is a highly effective catalyst for the oxidative Mannich reaction. The reaction proceeds via C-H oxidation of a tertiary amine followed by nucleophilic capture. This green transformation is conducted in protic solvent using inexpensive T-HYDRO (70% t-BuOOH in water). Synthetically valuable gamma-aminoalkyl butenolides are obtained.
Figures
References
-
-
For reviews of the Mannich reaction see: Kleinman ED. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 2. Pergamon; Oxford: 1991. p. 893. Arend M, Westermann B, Risch N. Angew. Chem., Int. Ed. 1998;37:1045. Royer J, Bonin M, Micouin L. Chem. Rev. 2004;104:2311.
-
-
- Speckamp WN, Moolenaar MJ. Tetrahedron. 2000;56:3817.
-
-
For metal-catalyzed oxidation of amines see: Murahashi S-I. Angew. Chem., Int. Ed. 1995;34:2443. Murahashi S-I, Imada Y. In: Transition Metals for Organic Synthesis. Beller M, Bolm C, editors. Vol. 2. Wiley-VCH; Weinheim: 2004. p. 497. Murahashi S-I, Komiya N. In: Modern Oxidation Methods. Bäckvall J-E, editor. Wiley-VCH; Weinheim: 2004. p. 165.
-
-
-
For oxidative Mannich reactions see: Li Z, Li C-J. J. Am. Chem. Soc. 2005;127:3672. Li ZP, Li CJ. Eur. J. Org. Chem. 2005:3173. and references therein. Murahashi S, Komiya N, Terai H, Nakae T. J. Am. Chem. Soc. 2003;125:15312. Murahashi S-I, Komiya N, Terai H. Angew. Chem., Int. Ed. 2005;44:6931.
-
-
-
For dirhodium-catalyzed oxidation see: Catino AJ, Forslund RE, Doyle MP. J. Am. Chem. Soc. 2004;126:13622. Catino AJ, Nichols JM, Choi H, Gottipamula S, Doyle MP. Org. Lett. 2005;7:5167.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous