Evaluation of prime/boost regimens using recombinant poxvirus/tyrosinase vaccines for the treatment of patients with metastatic melanoma
- PMID: 16638862
- PMCID: PMC2151202
- DOI: 10.1158/1078-0432.CCR-05-2061
Evaluation of prime/boost regimens using recombinant poxvirus/tyrosinase vaccines for the treatment of patients with metastatic melanoma
Abstract
Purpose: Two clinical trials were conducted to evaluate the clinical efficacy and immunologic impact of vaccination against the tyrosinase protein plus systemic interleukin 2 (IL-2) administration in patients with advanced metastatic melanoma.
Experimental design: Full-length tyrosinase was employed as an immunogen to induce diverse immunologic responses against a commonly expressed melanoma antigen. Heterologous prime/boost vaccination with recombinant vaccinia and fowlpox vectors encoding tyrosinase was first explored in a randomized three-arm phase II trial, in which vaccines were administered alone or concurrently with low-dose or high-dose IL-2. In a subsequent single cohort phase II trial, all patients received the same vaccines and high-dose IL-2 sequentially rather than concurrently.
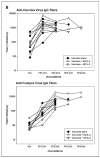
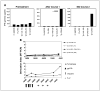
Results: Among a total of 64 patients treated on these trials, 8 objective partial responses (12.5%) were observed, all in patients receiving high-dose IL-2. Additional patients showed evidence of lesional regression (mixed tumor response) or overall regression that did not achieve partial response status (minor response). In vitro evidence of enhanced immunity against tyrosinase following protocol treatments was documented in 3 of 49 (6%) patients tested serologically, 3 of 23 (13%) patients tested for T-cell recognition of individual tyrosinase peptides, and 4 of 16 (25%) patients tested for T-cell recognition of full-length tyrosinase protein with real-time reverse transcription-PCR techniques.
Conclusions: Whereas prime/boost immunization with recombinant vaccinia and fowlpox viruses enhanced antityrosinase immunity in some patients with metastatic melanoma, it was ineffective alone in mediating clinical benefit, and in combination with IL-2 did not mediate clinical benefit significantly different from that expected from treatment with IL-2 alone.
Figures


Similar articles
-
Comparison of two cancer vaccines targeting tyrosinase: plasmid DNA and recombinant alphavirus replicon particles.Clin Cancer Res. 2005 Nov 15;11(22):8114-21. doi: 10.1158/1078-0432.CCR-05-1410. Clin Cancer Res. 2005. PMID: 16299244
-
A phase I vaccination study with tyrosinase in patients with stage II melanoma using recombinant modified vaccinia virus Ankara (MVA-hTyr).Cancer Immunol Immunother. 2005 May;54(5):453-67. doi: 10.1007/s00262-004-0616-7. Epub 2004 Dec 31. Cancer Immunol Immunother. 2005. PMID: 15627214 Free PMC article. Clinical Trial.
-
Intra-lymph node prime-boost vaccination against Melan A and tyrosinase for the treatment of metastatic melanoma: results of a phase 1 clinical trial.Clin Cancer Res. 2011 May 1;17(9):2987-96. doi: 10.1158/1078-0432.CCR-10-3272. Epub 2011 Mar 8. Clin Cancer Res. 2011. PMID: 21385924 Clinical Trial.
-
Local delivery of poxvirus vaccines for melanoma.Semin Cancer Biol. 2003 Dec;13(6):417-22. doi: 10.1016/j.semcancer.2003.09.005. Semin Cancer Biol. 2003. PMID: 15001160 Review.
-
Prospects and limitations of recombinant poxviruses for prostate cancer immunotherapy.Curr Opin Mol Ther. 1999 Aug;1(4):471-9. Curr Opin Mol Ther. 1999. PMID: 11713762 Review.
Cited by
-
Interleukin-13 receptor α2 DNA prime boost vaccine induces tumor immunity in murine tumor models.J Transl Med. 2010 Nov 10;8:116. doi: 10.1186/1479-5876-8-116. J Transl Med. 2010. PMID: 21067607 Free PMC article.
-
An integrated genome-wide approach to discover tumor-specific antigens as potential immunologic and clinical targets in cancer.Cancer Res. 2012 Dec 15;72(24):6351-61. doi: 10.1158/0008-5472.CAN-12-1656. Epub 2012 Nov 7. Cancer Res. 2012. PMID: 23135912 Free PMC article.
-
A systematic analysis on the clinical safety and efficacy of onco-virotherapy.Mol Ther Oncolytics. 2021 Oct 5;23:239-253. doi: 10.1016/j.omto.2021.09.008. eCollection 2021 Dec 17. Mol Ther Oncolytics. 2021. PMID: 34761104 Free PMC article.
-
Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus.Mol Ther. 2009 Oct;17(10):1814-21. doi: 10.1038/mt.2009.154. Epub 2009 Jul 14. Mol Ther. 2009. PMID: 19603003 Free PMC article.
-
Viral vector-based therapeutic cancer vaccines.Cancer J. 2011 Sep-Oct;17(5):359-71. doi: 10.1097/PPO.0b013e3182325e63. Cancer J. 2011. PMID: 21952287 Free PMC article. Review.
References
-
- Robbins PF, Kawakami Y. Human tumor antigens recognized by T cells. Curr Opin Immunol. 1996;8:628–36. - PubMed
-
- Kawakami Y, Robbins PF, Wang X, et al. Identification of new melanoma epitopes on melanosomal proteins recognized by tumor infiltrating T lymphocytes restricted by HLA-A1, -A2, and -A3 alleles. J Immunol. 1998;161:6985–92. - PubMed
-
- Robbins PF, El-Gamil M, Kawakami Y, et al. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994;54:3124–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

