Synthesis and pharmacological evaluation of novel 9- and 10-substituted cytisine derivatives. Nicotinic ligands of enhanced subtype selectivity
- PMID: 16640326
- PMCID: PMC2504867
- DOI: 10.1021/jm051196m
Synthesis and pharmacological evaluation of novel 9- and 10-substituted cytisine derivatives. Nicotinic ligands of enhanced subtype selectivity
Abstract
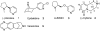
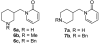
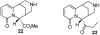
We report the synthesis and pharmacological properties of several cytisine derivatives. Among them, two 10-substituted derivatives showed much higher selectivities for the alpha4beta2 nAChR subtype in binding assays than cytisine. The 9-vinyl derivative was found to have a very similar agonist activity profile to that of cytisine.
Figures



References
-
- Holladay MW, Dart MJ, Lynch JK. Neuronal nicotinic acetylcholine receptors as targets for drug discovery. J. Med. Chem. 1997;40:4169–4194. - PubMed
-
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002;3:102–114. - PubMed
- Jensen AA, Frølund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 2005;48:4705–4745. - PubMed
-
- Hogg RC, Bertrand D. Nicotinic acetylcholine receptors as drug targets. Curr. Drug. Targets. CNS Neurol. Disord. 2004;3:123–130. - PubMed
- Clementi F, Fornasari D, Gotti C. Neuronal nicotinic acetylcholine receptors: from structure to therapeutics. Trends Pharmacol Sci. 2000;21:35–37. - PubMed
- Kellar KJ, Dâavila-Garcâia MI, Xiao Y. Pharmacology of neuronal nicotinic acetylcholine recceptors: effects of acute and chronic nicotine. Nicotine Tob. Res. 1999;1:S117–120. discussion S139-140. - PubMed
- Daly JW. Nicotinic Agonists, Antagonists and Modulators From Natural Sources. Cell. Mol. Neurobiol. 2005;25:513–551. - PMC - PubMed
-
- Millar NS. Assembly and subunit diversity of nicotinic acetylcholine receptors. Biochem. Soc. Trans. 2003;31:869–874. - PubMed
-
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog. Neurobiol. 2004;74:363–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

