Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized
- PMID: 16641091
- PMCID: PMC1464328
- DOI: 10.1073/pnas.0600605103
Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized
Abstract
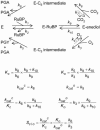
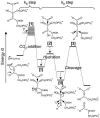
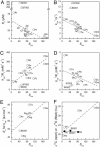
The cornerstone of autotrophy, the CO(2)-fixing enzyme, d-ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), is hamstrung by slow catalysis and confusion between CO(2) and O(2) as substrates, an "abominably perplexing" puzzle, in Darwin's parlance. Here we argue that these characteristics stem from difficulty in binding the featureless CO(2) molecule, which forces specificity for the gaseous substrate to be determined largely or completely in the transition state. We hypothesize that natural selection for greater CO(2)/O(2) specificity, in response to reducing atmospheric CO(2):O(2) ratios, has resulted in a transition state for CO(2) addition in which the CO(2) moiety closely resembles a carboxylate group. This maximizes the structural difference between the transition states for carboxylation and the competing oxygenation, allowing better differentiation between them. However, increasing structural similarity between the carboxylation transition state and its carboxyketone product exposes the carboxyketone to the strong binding required to stabilize the transition state and causes the carboxyketone intermediate to bind so tightly that its cleavage to products is slowed. We assert that all Rubiscos may be nearly perfectly adapted to the differing CO(2), O(2), and thermal conditions in their subcellular environments, optimizing this compromise between CO(2)/O(2) specificity and the maximum rate of catalytic turnover. Our hypothesis explains the feeble rate enhancement displayed by Rubisco in processing the exogenously supplied carboxyketone intermediate, compared with its nonenzymatic hydrolysis, and the positive correlation between CO(2)/O(2) specificity and (12)C/(13)C fractionation. It further predicts that, because a more product-like transition state is more ordered (decreased entropy), the effectiveness of this strategy will deteriorate with increasing temperature.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Comment in
-
A unified theory for the basis of the limitations of the primary reaction of photosynthetic CO(2) fixation: was Dr. Pangloss right?Proc Natl Acad Sci U S A. 2006 May 9;103(19):7203-4. doi: 10.1073/pnas.0602075103. Epub 2006 May 1. Proc Natl Acad Sci U S A. 2006. PMID: 16651526 Free PMC article. No abstract available.
References
-
- Ellis R. J. Trends Biochem. Sci. 1979;4:241–244.
-
- Andrews T. J., Lorimer G. H. In: The Biochemistry of Plants: A Comprehensive Treatise. Hatch M. D., Boardman N. K., editors. Vol. 10. New York: Academic; 1987. pp. 131–218.
-
- Spreitzer R. J., Salvucci M. E. Annu. Rev. Plant Biol. 2002;53:449–475. - PubMed
-
- Andrews T. J., Whitney S. M. Arch. Biochem. Biophys. 2003;414:159–169. - PubMed
-
- Lorimer G. H., Andrews T. J. Nature. 1973;243:359–360.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

