Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice
- PMID: 16641092
- PMCID: PMC1459012
- DOI: 10.1073/pnas.0602266103
Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice
Abstract
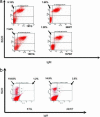
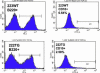
MicroRNAs (miRNAs) represent a newly discovered class of posttranscriptional regulatory noncoding small RNAs that bind to targeted mRNAs and either block their translation or initiate their degradation. miRNA profiling of hematopoietic lineages in humans and mice showed that some miRNAs are differentially expressed during hematopoietic development, suggesting a role in hematopoietic cell differentiation. In addition, recent studies suggest the involvement of miRNAs in the initiation and progression of cancer. miR155 and BIC, its host gene, have been reported to accumulate in human B cell lymphomas, especially in diffuse large B cell lymphomas, Hodgkin lymphomas, and certain types of Burkitt lymphomas. Here, we show that E(mu)-mmu-miR155 transgenic mice exhibit initially a preleukemic pre-B cell proliferation evident in spleen and bone marrow, followed by frank B cell malignancy. These findings indicate that the role of miR155 is to induce polyclonal expansion, favoring the capture of secondary genetic changes for full transformation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures






References
-
- Lee R. C., Feinbaum R. L., Ambros V. Cell. 1993;75:843–854. - PubMed
-
- Nairz K., Rottig C., Rintelen F., Zdobnov E., Moser M., Hafen E. Dev. Biol. 2006;291:314–324. - PubMed
-
- Naguibneva I., Ameyar-Zazoua M., Polesskaya A., Ait-Si-Ali S., Groisman R., Souidi M., Cuvellier S., Harel-Bellan A. Nat. Cell Biol. 2006;8:278–284. - PubMed
-
- Esau C., Davis S., Murray S. F., Yu X. X., Pandey S. K., Pear M., Watts L., Booten S. L., Graham M., McKay R., et al. Cell Metab. 2006;3:87–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

