Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation
- PMID: 16641105
- PMCID: PMC1458991
- DOI: 10.1073/pnas.0509719103
Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation
Abstract
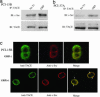
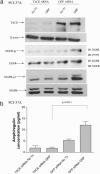
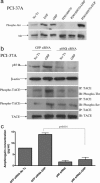
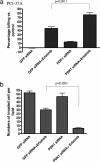
G protein-coupled receptors induce EGF receptor (EGFR) signaling, leading to the proliferation and invasion of cancer cells. Elucidation of the mechanism of EGFR activation by G protein-coupled receptors may identify new signaling paradigms. A gastrin-releasing peptide (GRP)/GRP receptor-mediated autocrine pathway was previously described in squamous cell carcinoma of head and neck. In the present study, we demonstrate that TNF-alpha converting enzyme (TACE), a disintegrin and metalloproteinse-17, undergoes a Src-dependent phosphorylation that regulates release of the EGFR ligand amphiregulin upon GRP treatment. Further investigation reveals the phosphatidylinositol 3-kinase (PI3-K) as the intermediate of c-Src and TACE, contributing to their association and TACE phosphorylation. Phosphoinositide-dependent kinase 1 (PDK1), a downstream target of PI3-K, has been identified as the previously undescribed kinase to directly phosphorylate TACE upon GRP treatment. These findings suggest a signaling cascade of GRP-Src-PI3-K-PDK1-TACE-amphiregulin-EGFR with multiple points of interaction, translocation, and phosphorylation. Furthermore, knockdown of PDK1 augmented the antitumor effects of the EGFR inhibitor erlotinib, indicating PDK1 as a therapeutic target to improve the clinical response to EGFR inhibitors.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


Similar articles
-
SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation, and invasion of head and neck cancer cells.Cancer Res. 2004 Sep 1;64(17):6166-73. doi: 10.1158/0008-5472.CAN-04-0504. Cancer Res. 2004. PMID: 15342401
-
Gastrin-releasing peptide activates Akt through the epidermal growth factor receptor pathway and abrogates the effect of gefitinib.Exp Cell Res. 2007 Apr 15;313(7):1361-72. doi: 10.1016/j.yexcr.2007.01.016. Epub 2007 Feb 3. Exp Cell Res. 2007. PMID: 17349623
-
TACE/ADAM-17: a component of the epidermal growth factor receptor axis and a promising therapeutic target in colorectal cancer.Clin Cancer Res. 2008 Feb 15;14(4):1182-91. doi: 10.1158/1078-0432.CCR-07-1216. Clin Cancer Res. 2008. PMID: 18281553 Free PMC article.
-
TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase.Ann N Y Acad Sci. 2003 May;995:22-38. doi: 10.1111/j.1749-6632.2003.tb03207.x. Ann N Y Acad Sci. 2003. PMID: 12814936 Review.
-
The ADAM17-amphiregulin-EGFR axis in mammary development and cancer.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):181-94. doi: 10.1007/s10911-008-9084-6. Epub 2008 May 10. J Mammary Gland Biol Neoplasia. 2008. PMID: 18470483 Free PMC article. Review.
Cited by
-
Profile of erlotinib and its potential in the treatment of advanced ovarian carcinoma.Onco Targets Ther. 2013 Apr 18;6:427-35. doi: 10.2147/OTT.S30373. Print 2013. Onco Targets Ther. 2013. PMID: 23723710 Free PMC article.
-
A biosensor for the activity of the "sheddase" TACE (ADAM17) reveals novel and cell type-specific mechanisms of TACE activation.Sci Signal. 2015 Feb 24;8(365):rs1. doi: 10.1126/scisignal.2005680. Sci Signal. 2015. PMID: 25714465 Free PMC article.
-
Relationships between serum HER2 ECD, TIMP-1 and clinical outcomes in Taiwanese breast cancer.World J Surg Oncol. 2012 Feb 17;10:42. doi: 10.1186/1477-7819-10-42. World J Surg Oncol. 2012. PMID: 22339939 Free PMC article.
-
Erlotinib, erlotinib-sulindac versus placebo: a randomized, double-blind, placebo-controlled window trial in operable head and neck cancer.Clin Cancer Res. 2014 Jun 15;20(12):3289-98. doi: 10.1158/1078-0432.CCR-13-3360. Epub 2014 Apr 11. Clin Cancer Res. 2014. PMID: 24727329 Free PMC article. Clinical Trial.
-
ADAM-17: the enzyme that does it all.Crit Rev Biochem Mol Biol. 2010 Apr;45(2):146-69. doi: 10.3109/10409231003628015. Crit Rev Biochem Mol Biol. 2010. PMID: 20184396 Free PMC article. Review.
References
-
- Lynch T. J., Bell D. W., Sordella R., Gurubhagavatula S., Okimoto R. A., Brannigan B. W., Harris P. L., Haserlat S. M., Supko J. G., Haluska F. G., et al. N. Engl. J. Med. 2004;350:2129–2139. - PubMed
-
- Paez J. G., Janne P. A., Lee J. C., Tracy S., Greulich H., Gabriel S., Herman P., Kaye F. J., Lindeman N., Boggon T. J., et al. Science. 2004;304:1497–1500. - PubMed
-
- Baselga J. Ann. Oncol. 2000;11(Suppl. 3):187–190. - PubMed
-
- Garber K. J. Natl. Cancer Inst. 2000;92:967–969. - PubMed
-
- Arteaga C. L., Baselga J. Cancer Cell. 2004;5:525–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

