GAP-independent termination of photoreceptor light response by excess gamma subunit of the cGMP-phosphodiesterase
- PMID: 16641226
- PMCID: PMC2852461
- DOI: 10.1523/JNEUROSCI.4775-05.2006
GAP-independent termination of photoreceptor light response by excess gamma subunit of the cGMP-phosphodiesterase
Abstract
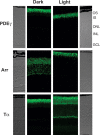
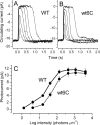
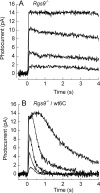
We have generated a mouse with rod photoreceptors overexpressing the gamma inhibitory subunit (PDE6gamma) of the photoreceptor G-protein effector cGMP phosphodiesterase (PDE6). PDE6gamma overexpression decreases the rate of rise of the rod response at dim intensities, indicating a reduction in the gain of transduction that may be the result of cytoplasmic PDE6gamma binding to activated transducin alpha GTP (Talpha-GTP) before the Talpha-GTP binds to endogenous PDE6gamma. Excess PDE6gamma also produces a marked acceleration in the falling phase of the light response and more rapid recovery of sensitivity and circulating current after prolonged light exposure. These effects are not mediated by accelerating GTP hydrolysis through the GAP (GTPase activating protein) complex, because the decay of the light response is also accelerated in rods that overexpress PDE6gamma but lack RGS9. Our results show that the PDE6gamma binding sites of PDE6 alpha and beta are accessible to excess (presumably cytoplasmic) PDE6gamma in the light, once endogenous PDE6gamma has been displaced from its binding site by Talpha-GTP. They also suggest that in the presence of Talpha-GTP, the PDE6gamma remains attached to the rest of the PDE6 molecule, but after conversion of Talpha-GTP to Talpha-GDP, the PDE6gamma may dissociate from the PDE6 and exchange with a cytoplasmic pool. This pool may exist even in wild-type rods and may explain the decay of rod photoresponses in the presence of nonhydrolyzable analogs of GTP.
Figures




References
-
- Angleson J, Wensel T (1993). A GTPase-accelerating factor for transducin, distinct from its effector cGMP phosphodiesterase, in rod outer segment membranes. Neuron 11:939–949. - PubMed
-
- Angleson J, Wensel T (1994). Enhancement of rod outer segment GTPase accelerating protein activity by the inhibitory subunit of cGMP phosphodiesterase. J Biol Chem 269:16290–16296. - PubMed
-
- Antonny B, Otto-Bruc A, Chabre M, Vuong TM (1993). GTP hydrolysis by purified alpha-subunit of transducin and its complex with the cyclic GMP phosphodiesterase inhibitor. Biochemistry 32:8646–8653. - PubMed
-
- Arshavsky VY, Dumke CL, Zhu Y, Artemyev NO, Skiba NP, Hamm HE, Bownds MD (1994). Regulation of transducin GTPase activity in bovine rod outer segments. J Biol Chem 269:19882–19887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
