Interaction of Kv3 potassium channels and resurgent sodium current influences the rate of spontaneous firing of Purkinje neurons
- PMID: 16641240
- PMCID: PMC6674064
- DOI: 10.1523/JNEUROSCI.5204-05.2006
Interaction of Kv3 potassium channels and resurgent sodium current influences the rate of spontaneous firing of Purkinje neurons
Abstract
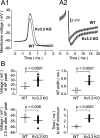
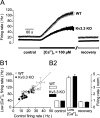
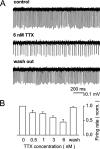
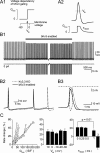
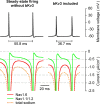
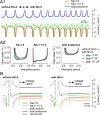
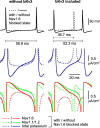
Purkinje neurons spontaneously generate action potentials in the absence of synaptic drive and thereby exert a tonic, yet plastic, input to their target cells in the deep cerebellar nuclei. Purkinje neurons express two ionic currents with biophysical properties that are specialized for high-frequency firing: resurgent sodium currents and potassium currents mediated by Kv3.3. How these ionic currents determine the intrinsic activity of Purkinje neurons has only partially been understood. Purkinje neurons from mutant mice lacking Kv3.3 have a reduced rate of spontaneous firing. Dynamic-clamp recordings demonstrated that normal firing rates are rescued by inserting artificial Kv3 currents into Kv3.3 knock-out Purkinje neurons. Numerical simulations indicated that Kv3.3 increases the spontaneous firing rate via cooperation with resurgent sodium currents. We conclude that the rate of spontaneous action potential firing of Purkinje neurons is controlled by the interaction of Kv3.3 potassium currents and resurgent sodium currents.
Figures


References
-
- Atzori M, Lau D, Tansey EP, Chow A, Ozaita A, Rudy B, McBain CJ (2000). H2 histamine receptor-phosphorylation of Kv3.2 modulates interneuron fast spiking. Nat Neurosci 3:791–798. - PubMed
-
- Bal T, McCormick DA (1997). Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I(h). J Neurophysiol 77:3145–3156. - PubMed
-
- Behnisch T, Matsushita S, Knöpfel T (2004). Imaging of gene expression during long-term potentiation. NeuroReport 15:2039–2043. - PubMed
-
- Benardo LS, Foster RE (1986). Oscillatory behavior in inferior olive neurons: mechanism, modulation, cell aggregates. Brain Res Bull 17:773–784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
