Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy
- PMID: 16641241
- PMCID: PMC6674073
- DOI: 10.1523/JNEUROSCI.0064-06.2006
Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy
Abstract
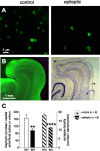
Temporal lobe epilepsy is the most common type of epilepsy in adults, and its pathophysiology remains unclear. Layer II stellate cells of the entorhinal cortex, which are hyperexcitable in animal models of temporal lobe epilepsy, provide the predominant synaptic input to the hippocampal dentate gyrus. Previous studies have ascribed the hyperexcitability of layer II stellate cells to GABAergic interneurons becoming "dormant" after disconnection from their excitatory synaptic inputs, which has been reported to occur during preferential loss of layer III pyramidal cells. We used whole-cell recording from slices of entorhinal cortex in pilocarpine-treated epileptic rats to test the dormant interneuron hypothesis. Hyperexcitability appeared as multiple action potentials and prolonged depolarizations evoked in layer II stellate cells of epileptic rats but not controls. However, blockade of glutamatergic synaptic transmission caused similar percentage reductions in the frequency of spontaneous IPSCs in layer II stellate cells of control and epileptic rats, suggesting similar levels of excitatory synaptic input to GABAergic interneurons. Direct recordings and biocytin labeling revealed two major types of interneurons in layer III whose excitatory synaptic drive in epileptic animals was undiminished. Interneurons in layer III did not appear to be dormant; therefore, we tested whether loss of GABAergic synapses might underlie hyperexcitability of layer II stellate cells. Stereological evidence of fewer GABAergic interneurons, fewer gephyrin-immunoreactive punctae, and reduced frequency of spontaneous IPSCs and miniature IPSCs (recorded in tetrodotoxin) confirmed that layer II stellate cell hyperexcitability is attributable, at least in part, to reduced inhibitory synaptic input.
Figures






References
-
- Agrawal N, Alonso A, Ragsdale DS (2003). Increased persistent sodium currents in rat entorhinal cortex layer V neurons in a post-status epilepticus model of temporal lobe epilepsy. Epilepsia 44:1601–1604. - PubMed
-
- Alonso A, Klink R (1993). Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol 70:128–143. - PubMed
-
- Bartolomei F, Khalil M, Wendling F, Sontheimer A, Régis J, Ranjeva J-P, Guye M, Chauvel P (2005). Entorhinal cortex involvement in human mesial temporal lobe epilepsy: an electrophysiological and volumetric study. Epilepsia 45:677–687. - PubMed
-
- Bear J, Fountain NB, Lothman EW (1996). Responses of the superficial entorhinal cortex in vitro in slices from naive and chronically epileptic rats. J Neurophysiol 76:2928–2940. - PubMed
-
- Bekenstein JW, Lothman EW (1993). Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science 259:97–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
