Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat
- PMID: 16641248
- PMCID: PMC6674074
- DOI: 10.1523/JNEUROSCI.5539-05.2006
Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat
Abstract
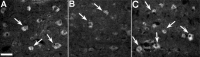
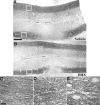
Spinal cord injury (SCI) is a cause of major neurological disability, and no satisfactory treatment is currently available. Evidence suggests that polyunsaturated fatty acids (PUFAs) could target some of the pathological mechanisms that underlie damage after SCI. We examined the effects of treatment with PUFAs after lateral spinal cord hemisection in the rat. The omega-3 PUFAs alpha-linolenic acid and docosahexaenoic acid (DHA) injected 30 min after injury induced significantly improved locomotor performance and neuroprotection, including decreased lesion size and apoptosis and increased neuronal and oligodendrocyte survival. Evidence showing a decrease in RNA/DNA oxidation suggests that the neuroprotective effect of omega-3 PUFAs involved a significant antioxidant function. In contrast, animals treated with arachidonic acid, an omega-6 PUFA, had a significantly worse outcome than controls. We confirmed the neuroprotective effect of omega-3 PUFAs by examining the effects of DHA treatment after spinal cord compression injury. Results indicated that DHA administered 30 min after spinal cord compression not only greatly increased survival of neurons but also resulted in significantly better locomotor performance for up to 6 weeks after injury. This report shows a striking difference in efficacy between the effects of treatment with omega-3 and omega-6 PUFAs on the outcome of SCI, with omega-3 PUFAs being neuroprotective and omega-6 PUFAs having a damaging effect. Given the proven clinical safety of omega-3 PUFAs, our observations show that these PUFAs have significant therapeutic potential in SCI. In contrast, the use of preparations enriched in omega-6 PUFAs after injury could worsen outcome after SCI.
Figures






References
-
- Basso DM, Beattie MS, Bresnahan JC (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139:244–256. - PubMed
-
- Beattie MS, Farooqui AA, Bresnahan JC (2000). Rev of current evidence for apoptosis after spinal cord injury. J Neurotrauma 17:915–925. - PubMed
-
- Blondeau N, Widmann C, Lazdunski M, Heurteaux C (2002). Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience 109:231–241. - PubMed
-
- Calder PC (2003). Long-chain n-3 fatty acids and inflammation: potential application in surgical and trauma patients. Braz J Med Biol Res 36:433–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
