Tax interacts with P-TEFb in a novel manner to stimulate human T-lymphotropic virus type 1 transcription
- PMID: 16641271
- PMCID: PMC1472077
- DOI: 10.1128/JVI.80.10.4781-4791.2006
Tax interacts with P-TEFb in a novel manner to stimulate human T-lymphotropic virus type 1 transcription
Abstract
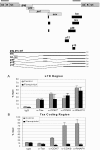
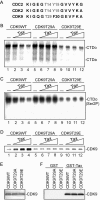
Human T-lymphotropic virus type 1 (HTLV-1) encodes a transcriptional activator, Tax, whose function is essential for viral transcription and replication. Tax transactivates the viral long-terminal repeat through a series of protein-protein interactions which facilitate CREB and CBP/p300 binding. In addition, Tax dissociates transcription repressor histone deacetylase 1 interaction with the CREB response element. The subsequent events through which Tax interacts and communicates with RNA polymerase II and cyclin-dependent kinases (CDKs) are not clearly understood. Here we present evidence that Tax recruits positive transcription elongation factor b (P-TEFb) (CDK9/cyclin T1) to the viral promoter. This recruitment likely involves protein-protein interactions since Tax associates with P-TEFb in vitro as demonstrated by glutathione S-transferase fusion protein pull-down assays and in vivo as shown by co-immunoprecipitation assays. Functionally, small interfering RNA directed toward CDK9 inhibited Tax transactivation in transient assays. Consistent with these findings, the depletion of CDK9 from nuclear extracts inhibited Tax transactivation in vitro. Reconstitution of the reaction with wild-type P-TEFb, but not a kinase-dead mutant, recovered HTLV-1 transcription. Moreover, the addition of the CDK9 inhibitor flavopiridol blocked Tax transactivation in vitro and in vivo. Interestingly, we found that Tax regulates CDK9 kinase activity through a novel autophosphorylation pathway.
Figures





References
-
- Astier-Gin, T., J. P. Portail, F. Lafond, and B. Guillemain. 1995. Identification of HTLV-I- or HTLV-II-producing cells by cocultivation with BHK-21 cells stably transfected with a LTR-lacZ gene construct. J. Virol. Methods 51:19-29. - PubMed
-
- Bex, F., and R. B. Gaynor. 1998. Regulation of gene expression by HTLV-I Tax protein. Methods 16:83-94. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

