Rotavirus viremia and extraintestinal viral infection in the neonatal rat model
- PMID: 16641274
- PMCID: PMC1472071
- DOI: 10.1128/JVI.80.10.4820-4832.2006
Rotavirus viremia and extraintestinal viral infection in the neonatal rat model
Abstract
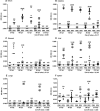
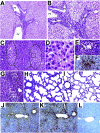
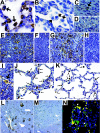
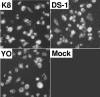
Rotaviruses infect mature, differentiated enterocytes of the small intestine and, by an unknown mechanism, escape the gastrointestinal tract and cause viremia. The neonatal rat model of rotavirus infection was used to determine the kinetics of viremia, spread, and pathology of rotavirus in extraintestinal organs. Five-day-old rat pups were inoculated intragastrically with an animal (RRV) or human (HAL1166) rotavirus or phosphate-buffered saline. Blood was collected from a subset of rat pups, and following perfusion to remove residual blood, organs were removed and homogenized to analyze rotavirus-specific antigen by enzyme-linked immunosorbent assay and infectious rotavirus by fluorescent focus assay or fixed in formalin for histology and immunohistochemistry. Viremia was detected following rotavirus infection with RRV and HAL1166. The RRV 50% antigenemia dose was 1.8 x 10(3) PFU, and the 50% diarrhea dose was 7.7 x 10(5) PFU, indicating that infection and viremia occurred in the absence of diarrhea and that detecting rotavirus antigen in the blood was a more sensitive measure of infection than diarrhea. Rotavirus antigens and infectious virus were detected in multiple organs (stomach, intestines, liver, lungs, spleen, kidneys, pancreas, thymus, and bladder). Histopathological changes due to rotavirus infection included acute inflammation of the portal tract and bile duct, microsteatosis, necrosis, and inflammatory cell infiltrates in the parenchymas of the liver and lungs. Colocalization of structural and nonstructural proteins with histopathology in the liver and lungs indicated that the histological changes observed were due to rotavirus infection and replication. Replicating rotavirus was also detected in macrophages in the lungs and blood vessels, indicating a possible mechanism of rotavirus dissemination. Extraintestinal infectious rotavirus, but not diarrhea, was observed in the presence of passively or actively acquired rotavirus-specific antibody. These findings alter the previously accepted concept of rotavirus pathogenesis to include not only gastroenteritis but also viremia, and they indicate that rotavirus could cause a broad array of systemic diseases in a number of different organs.
Figures
References
-
- Ball, J. M., P. Tian, C. Q. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101-104. - PubMed
-
- Blutt, S. E., C. D. Kirkwood, V. Parreño, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. - PubMed
-
- Boshuizen, J. A., J. H. J. Reimerink, A. M. Korteland-van Male, V. J. J. van Ham, M. P. G. Koopmans, H. A. Büller, J. Dekker, and A. W. C. Einerhand. 2003. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J. Virol. 77:13005-13016. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

