Adeno-associated virus type 2 increases proteosome-dependent degradation of p21WAF1 in a human papillomavirus type 31b-positive cervical carcinoma line
- PMID: 16641284
- PMCID: PMC1472069
- DOI: 10.1128/JVI.80.10.4927-4939.2006
Adeno-associated virus type 2 increases proteosome-dependent degradation of p21WAF1 in a human papillomavirus type 31b-positive cervical carcinoma line
Abstract
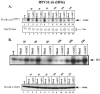
Adeno-associated virus type 2 (AAV2) seropositivity is negatively correlated with the development of human papillomavirus (HPV)-associated cervical cancer. We have begun analysis of the molecular mechanisms underlying AAV2-mediated onco-suppression through cell cycle regulation in HPV-infected keratinocytes isolated from a low-grade cervical lesion. AAV2 superinfection of HPV type 31b (HPV31b)-positive cells at early times postinfection resulted in degradation of the cyclin-dependent kinase (CDK) inhibitor p21(WAF1) protein in a proteosome-dependent manner. Downstream consequences of lowering p21(WAF1) levels included a proportional loss of cyclin E/CDK2 complexes bound to p21(WAF1). The loss of stable p21(WAF1)/cyclin E/CDK2 complexes coincided with an increase in CDK2-associated kinase activity and cyclin E levels. Both events have the potential to enhance the G(1)/S transition point mediated by active cyclin E/CDK2 complexes. Concurrently, cyclin A and E2F levels were decreased, conditions reminiscent of delayed entrance into the S phase of the cell cycle. On the other hand, infection of primary human foreskin keratinocytes with AAV2 resulted in upregulation of p21(WAF1) protein levels, reminiscent of a block in G(1) phase progression. We propose that by down regulating p21(WAF1), AAV2 initiates cell cycle activities leading to enhanced G(1)/S phase-like conditions which may be favorable for AAV2-specific functions and may lead to downstream interference with HPV-associated cervical cancer progression.
Figures

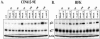


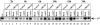



References
-
- Adams, P. D., and W. G. Kaelin, Jr. 1996. The cellular effects of E2F overexpression. Curr. Top. Microbiol. Immunol. 208:79-93. - PubMed
-
- Bantel-Schaal, U. 1995. Growth properties of a human melanoma cell line are altered by adeno-associated parvovirus type 2. Int. J. Cancer 60:269-274. - PubMed
-
- Bantel-Schaal, U., and H. zur Hausen. 1984. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology 134:52-63. - PubMed
-
- Batchu, R. B., M. A. Shammas, J. Y. Wang, J. Freeman, N. Rosen, and N. C. Munshi. 2002. Adeno-associated virus protects the retinoblastoma family of proteins from adenoviral-induced functional inactivation. Cancer Res. 62:2982-2985. - PubMed
-
- Batchu, R. B., M. A. Shammas, J. Y. Wang, and N. C. Munshi. 2001. Dual level inhibition of E2F-1 activity by adeno-associated virus Rep78. J. Biol. Chem. 276:24315-24322. - PubMed

