Cellular splicing and transcription regulatory protein p32 represses adenovirus major late transcription and causes hyperphosphorylation of RNA polymerase II
- PMID: 16641292
- PMCID: PMC1472059
- DOI: 10.1128/JVI.80.10.5010-5020.2006
Cellular splicing and transcription regulatory protein p32 represses adenovirus major late transcription and causes hyperphosphorylation of RNA polymerase II
Abstract
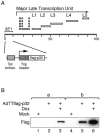
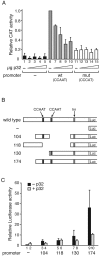
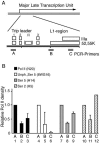
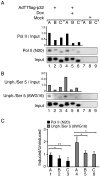
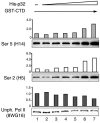
The cellular protein p32 is a multifunctional protein, which has been shown to interact with a large number of cellular and viral proteins and to regulate several important activities like transcription and RNA splicing. We have previously shown that p32 regulates RNA splicing by binding and inhibiting the essential SR protein ASF/SF2. To determine whether p32 also functions as a regulator of splicing in virus-infected cells, we constructed a recombinant adenovirus expressing p32 under the transcriptional control of an inducible promoter. Much to our surprise the results showed that p32 overexpression effectively blocked mRNA and protein expression from the adenovirus major late transcription unit (MLTU). Interestingly, the p32-mediated inhibition of MLTU transcription was accompanied by an approximately 4.5-fold increase in Ser 5 phosphorylation and an approximately 2-fold increase in Ser 2 phosphorylation of the carboxy-terminal domain (CTD). Further, in p32-overexpressing cells the efficiency of RNA polymerase elongation was reduced approximately twofold, resulting in a decrease in the number of polymerase molecules that reached the end of the major late L1 transcription unit. We further show that p32 stimulates CTD phosphorylation in vitro. The inhibitory effect of p32 on MLTU transcription appears to require the CAAT box element in the major late promoter, suggesting that p32 may become tethered to the MLTU via an interaction with the CAAT box binding transcription factor.
Figures




Similar articles
-
The chaperone protein p32 stabilizes HIV-1 Tat and strengthens the p-TEFb/RNAPII/TAR complex promoting HIV transcription elongation.Proc Natl Acad Sci U S A. 2023 Jan 3;120(1):e2217476120. doi: 10.1073/pnas.2217476120. Epub 2022 Dec 30. Proc Natl Acad Sci U S A. 2023. PMID: 36584296 Free PMC article.
-
The splicing factor-associated protein, p32, regulates RNA splicing by inhibiting ASF/SF2 RNA binding and phosphorylation.EMBO J. 1999 Feb 15;18(4):1014-24. doi: 10.1093/emboj/18.4.1014. EMBO J. 1999. PMID: 10022843 Free PMC article.
-
Overexpression of essential splicing factor ASF/SF2 blocks the temporal shift in adenovirus pre-mRNA splicing and reduces virus progeny formation.J Virol. 2000 Oct;74(19):9002-9. doi: 10.1128/jvi.74.19.9002-9009.2000. J Virol. 2000. PMID: 10982344 Free PMC article.
-
hnRNP U inhibits carboxy-terminal domain phosphorylation by TFIIH and represses RNA polymerase II elongation.Mol Cell Biol. 1999 Oct;19(10):6833-44. doi: 10.1128/MCB.19.10.6833. Mol Cell Biol. 1999. PMID: 10490622 Free PMC article.
-
In vitro interaction between human immunodeficiency virus type 1 Rev protein and splicing factor ASF/SF2-associated protein, p32.J Biol Chem. 1996 Apr 26;271(17):10066-72. doi: 10.1074/jbc.271.17.10066. J Biol Chem. 1996. PMID: 8626563
Cited by
-
Attenuated strains of influenza A viruses do not induce degradation of RNA polymerase II.J Virol. 2009 Nov;83(21):11166-74. doi: 10.1128/JVI.01439-09. Epub 2009 Aug 19. J Virol. 2009. PMID: 19692472 Free PMC article.
-
SLE: Novel Postulates for Therapeutic Options.Front Immunol. 2020 Oct 7;11:583853. doi: 10.3389/fimmu.2020.583853. eCollection 2020. Front Immunol. 2020. PMID: 33117397 Free PMC article. Review.
-
Association of the influenza virus RNA polymerase subunit PB2 with the host chaperonin CCT.J Virol. 2010 Sep;84(17):8691-9. doi: 10.1128/JVI.00813-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573828 Free PMC article.
-
Interactions between RNA-binding proteins and P32 homologues in trypanosomes and human cells.Curr Genet. 2016 Feb;62(1):203-12. doi: 10.1007/s00294-015-0519-5. Epub 2015 Sep 18. Curr Genet. 2016. PMID: 26385742
-
The chaperone protein p32 stabilizes HIV-1 Tat and strengthens the p-TEFb/RNAPII/TAR complex promoting HIV transcription elongation.Proc Natl Acad Sci U S A. 2023 Jan 3;120(1):e2217476120. doi: 10.1073/pnas.2217476120. Epub 2022 Dec 30. Proc Natl Acad Sci U S A. 2023. PMID: 36584296 Free PMC article.
References
-
- Abbott, K. L., J. Archambault, H. Xiao, B. D. Nguyen, R. G. Roeder, J. Greenblatt, J. G. Omichinski, and P. Legault. 2005. Interactions of the HIV-1 Tat and RAP74 proteins with the RNA polymerase II CTD phosphatase FCP1. Biochemistry 44:2716-2731. - PubMed
-
- Ahn, S. H., M. Kim, and S. Buratowski. 2004. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol. Cell 13:67-76. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
-
- Berenjian, S., and G. Akusjärvi. 2006. Binary AdEasy vector systems designed for Tet-ON or Tet-OFF regulated control of transgene expression. Virus Res. 115:116-123. - PubMed
-
- Chambers, R. S., B. Q. Wang, Z. F. Burton, and M. E. Dahmus. 1995. The activity of COOH-terminal domain phosphatase is regulated by a docking site on RNA polymerase II and by the general transcription factors IIF and IIB. J. Biol. Chem. 270:14962-14969. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

