The Caenorhabditis elegans homologue of deleted in azoospermia is involved in the sperm/oocyte switch
- PMID: 16641369
- PMCID: PMC1483047
- DOI: 10.1091/mbc.e05-11-1067
The Caenorhabditis elegans homologue of deleted in azoospermia is involved in the sperm/oocyte switch
Abstract
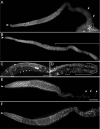
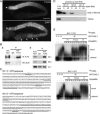
The Deleted in Azoospermia (DAZ) gene family encodes putative translational activators that are required for meiosis and other aspects of gametogenesis in animals. The single Caenorhabditis elegans homologue of DAZ, daz-1, is an essential factor for female meiosis. Here, we show that daz-1 is important for the switch from spermatogenesis to oogenesis (the sperm/oocyte switch), which is an essential step for the hermaphrodite germline to produce oocytes. RNA interference of the daz-1 orthologue in a related nematode, Caenorhabditis briggsae, resulted in a complete loss of the sperm/oocyte switch. The C. elegans hermaphrodite deficient in daz-1 also revealed a failure in the sperm/oocyte switch if the genetic background was conditional masculinization of germline. DAZ-1 could bind specifically to mRNAs encoding the FBF proteins, which are translational regulators for the sperm/oocyte switch and germ stem cell proliferation. Expression of the FBF proteins seemed to be lowered in the daz-1 mutant at the stage for the sperm/oocyte switch. Conversely, a mutation in gld-3, a gene that functionally counteracts FBF, could partially restore oogenesis in the daz-1 mutant. Together, we propose that daz-1 plays a role upstream of the pathway for germ cell sex determination.
Figures




Similar articles
-
Caenorhabditis elegans homologue of the human azoospermia factor DAZ is required for oogenesis but not for spermatogenesis.Development. 2000 Mar;127(5):1069-79. doi: 10.1242/dev.127.5.1069. Development. 2000. PMID: 10662646
-
NANOS-3 and FBF proteins physically interact to control the sperm-oocyte switch in Caenorhabditis elegans.Curr Biol. 1999 Sep 23;9(18):1009-18. doi: 10.1016/s0960-9822(99)80449-7. Curr Biol. 1999. PMID: 10508609
-
Redundant control of the Caenorhabditis elegans sperm/oocyte switch by PUF-8 and FBF-1, two distinct PUF RNA-binding proteins.Proc Natl Acad Sci U S A. 2005 Aug 2;102(31):10893-7. doi: 10.1073/pnas.0504593102. Epub 2005 Jul 21. Proc Natl Acad Sci U S A. 2005. PMID: 16037210 Free PMC article.
-
RNA and sex determination in Caenorhabditis elegans. Post-transcriptional regulation of the sex-determining tra-2 and fem-3 mRNAs in the Caenorhabditis elegans hermaphrodite.EMBO Rep. 2001 Oct;2(10):899-904. doi: 10.1093/embo-reports/kve209. EMBO Rep. 2001. PMID: 11600454 Free PMC article. Review.
-
Role of PUF-8/PUF protein in stem cell control, sperm-oocyte decision and cell fate reprogramming.J Cell Physiol. 2014 Oct;229(10):1306-11. doi: 10.1002/jcp.24618. J Cell Physiol. 2014. PMID: 24638209 Review.
Cited by
-
The role of RNA-binding proteins in orchestrating germline development in Caenorhabditis elegans.Front Cell Dev Biol. 2023 Jan 4;10:1094295. doi: 10.3389/fcell.2022.1094295. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36684428 Free PMC article. Review.
-
The TRIM-NHL protein LIN-41 and the OMA RNA-binding proteins antagonistically control the prophase-to-metaphase transition and growth of Caenorhabditis elegans oocytes.Genetics. 2014 Dec;198(4):1535-58. doi: 10.1534/genetics.114.168831. Epub 2014 Sep 26. Genetics. 2014. PMID: 25261698 Free PMC article.
-
Chapter 3. Caenorhabditis nematodes as a model for the adaptive evolution of germ cells.Curr Top Dev Biol. 2009;86:43-66. doi: 10.1016/S0070-2153(09)01003-5. Curr Top Dev Biol. 2009. PMID: 19361689 Free PMC article. Review.
-
Identification of proteins and miRNAs that specifically bind an mRNA in vivo.Nat Commun. 2019 Sep 16;10(1):4205. doi: 10.1038/s41467-019-12050-7. Nat Commun. 2019. PMID: 31527589 Free PMC article.
-
Differential conservation and divergence of fertility genes boule and dazl in the rainbow trout.PLoS One. 2011 Jan 6;6(1):e15910. doi: 10.1371/journal.pone.0015910. PLoS One. 2011. PMID: 21253610 Free PMC article.
References
-
- Ahringer J., Kimble J. Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3′ untranslated region. Nature. 1991;349:346–348. - PubMed
-
- Ashcroft N. R., Kosinski M. E., Wickramasinghe D., Donovan P. J., Golden A. The four cdc25 genes from the nematode Caenorhabditis elegans. Gene. 1998;214:59–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

