Multiple sequence elements facilitate Chp Rho GTPase subcellular location, membrane association, and transforming activity
- PMID: 16641371
- PMCID: PMC1483044
- DOI: 10.1091/mbc.e05-09-0896
Multiple sequence elements facilitate Chp Rho GTPase subcellular location, membrane association, and transforming activity
Abstract
Cdc42 homologous protein (Chp) is a member of the Rho family of small GTPases and shares significant sequence and functional similarity with Cdc42. However, unlike classical Rho GTPases, we recently found that Chp depends on palmitoylation, rather than prenylation, for association with cellular membranes. Because palmitoylation alone is typically not sufficient to promote membrane association, we evaluated the possibility that other carboxy-terminal residues facilitate Chp subcellular association with membranes. We found that Chp membrane association and transforming activity was dependent on the integrity of a stretch of basic amino acids in the carboxy terminus of Chp and that the basic amino acids were not simply part of a palmitoyl acyltransferase recognition motif. We also determined that the 11 carboxy-terminal residues alone were sufficient to promote Chp plasma and endomembrane association. Interestingly, stimulation with tumor necrosis factor-alpha activated only endomembrane-associated Chp. Finally, we found that Chp membrane association was not disrupted by Rho guanine nucleotide dissociation inhibitory proteins, which are negative regulators of Cdc42 membrane association and biological activity. In summary, the unique carboxy-terminal sequence elements that promote Chp subcellular location and function expand the complexity of mechanisms by which the cellular functions of Rho GTPases are regulated.
Figures



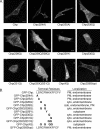



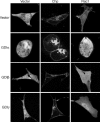

References
-
- Allal C., Favre G., Couderc B., Salicio S., Sixou S., Hamilton A. D., Sebti S. M., Lajoie-Mazenc I., Pradines A. RhoA prenylation is required for promotion of cell growth and transformation and cytoskeleton organization but not for induction of serum response element transcription. J. Biol. Chem. 2000;275:31001–31008. - PubMed
-
- Aronheim A., Broder Y. C., Cohen A., Fritsch A., Belisle B., Abo A. Chp, a homologue of the GTPase Cdc42Hs, activates the JNK pathway and is implicated in reorganizing the actin cytoskeleton. Curr. Biol. 1998;8:1125–1128. - PubMed
-
- Berzat A. C., Buss J. E., Chenette E. J., Weinbaum C. A., Shutes A., Der C. J., Minden A., Cox A. D. Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J. Biol. Chem. 2005;280:33055–33065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

