Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells
- PMID: 16641372
- PMCID: PMC1483048
- DOI: 10.1091/mbc.e05-08-0799
Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells
Abstract
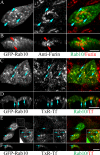
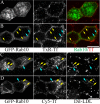
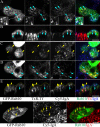
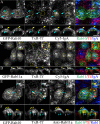
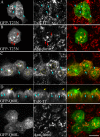
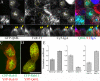
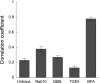
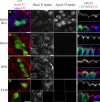
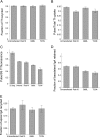
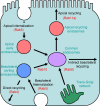
Rab10, a protein originally isolated from Madin-Darby Canine Kidney (MDCK) epithelial cells, belongs to a family of Rab proteins that includes Rab8 and Rab13. Although both Rab8 and Rab13 have been found to mediate polarized membrane transport, the function of Rab10 in mammalian cells has not yet been established. We have used quantitative confocal microscopy of polarized MDCK cells expressing GFP chimeras of wild-type and mutant forms of Rab10 to analyze the function of Rab10 in polarized cells. These studies demonstrate that Rab10 is specifically associated with the common endosomes of MDCK cells, accessible to endocytic probes internalized from either the apical or basolateral plasma membrane domains. Expression of mutant Rab10 defective for either GTP hydrolysis or GTP binding increased recycling from early compartments on the basolateral endocytic pathway without affecting recycling from later compartments or the apical recycling pathway. These results suggest that Rab10 mediates transport from basolateral sorting endosomes to common endosomes.
Figures


References
-
- Ali B., Seabra M. Targeting of Rab GTPases to cellular membranes. Biochem. Soc. Trans. 2005;33:652–656. - PubMed
-
- Baravelle G., Schober D., Huber M., Bayer N., Murphy R., Fuchs R. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole and low temperature. Cell Tissue Res. 2005;320:99–113. - PubMed
-
- Barbacid M. Ras genes. Annu. Rev. Biochem. 1987;56:779–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

