Cellular accumulation and activity of quinolones in ciprofloxacin-resistant J774 macrophages
- PMID: 16641436
- PMCID: PMC1472235
- DOI: 10.1128/AAC.50.5.1689-1695.2006
Cellular accumulation and activity of quinolones in ciprofloxacin-resistant J774 macrophages
Abstract
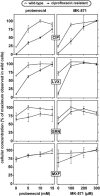
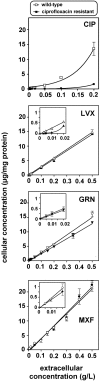
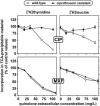
Ciprofloxacin is the substrate for a multidrug resistance-related protein (MRP)-like multidrug transporter in J774 mouse macrophages, which also modestly affects levofloxacin but only marginally affects garenoxacin and moxifloxacin (J.-M. Michot et al., Antimicrob. Agents Chemother. 49:2429-2437, 2005). Two clones of ciprofloxacin-resistant cells were obtained by a stepwise increase in drug concentration (from 34 to 51 to 68 mg/liter) in the culture fluid. Compared to wild-type cells, ciprofloxacin-resistant cells showed (i) a markedly reduced ciprofloxacin accumulation (12% of control) and (ii) a two- to threefold lower sensitivity to the enhancing effect exerted by MRP-inhibitors (probenecid and MK571) on ciprofloxacin accumulation or by ciprofloxacin itself. ATP-depletion brought ciprofloxacin accumulation to similarly high levels in both wild-type and ciprofloxacin-resistant cells. Garenoxacin and moxifloxacin accumulation remained unaffected, and levofloxacin showed an intermediate behavior. DNA and protein synthesis were not impaired in ciprofloxacin-resistant cells for ciprofloxacin concentrations up to 100 mg/liter (approximately 85 and 55% inhibition, respectively, in wild-type cells). In Listeria monocytogenes-infected ciprofloxacin-resistant cells, 12-fold higher extracellular concentrations of ciprofloxacin were needed to show a bacteriostatic effect in comparison with wild-type cells. The data suggest that the resistance mechanism is mediated by an overexpression and/or increased activity of the MRP-like ciprofloxacin transporter expressed at a basal level in wild-type J774 macrophages, which modulates both the intracellular pharmacokinetics and activity of ciprofloxacin.
Figures
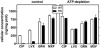

References
-
- Cao, C., T. H. Steinberg, H. C. Neu, D. Cohen, S. B. Horwitz, S. Hickman, and S. C. Silverstein. 1993. Probenecid-resistant J774 cell expression of enhanced organic anion transport by a mechanism distinct from multidrug resistance. Infect. Agents Dis. 2:193-200. - PubMed
-
- Carlier, M. B., B. Scorneaux, A. Zenebergh, J. F. Desnottes, and P. M. Tulkens. 1990. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J. Antimicrob. Chemother. 26(Suppl. B):27-39. - PubMed
-
- Deng, L., S. Tatebe, Y. C. Lin-Lee, T. Ishikawa, and M. T. Kuo. 2002. MDR and MRP gene families as cellular determinant factors for resistance to clinical anticancer agents. Cancer Treat. Res. 112:49-66. - PubMed
-
- Eriksson, E., A. Forsgren, and K. Riesbeck. 2003. Several gene programs are induced in ciprofloxacin-treated human lymphocytes as revealed by microarray analysis. J. Leukoc. Biol. 74:456-463. - PubMed
-
- Gollapudi, S., F. Thadepalli, C. H. Kim, and S. Gupta. 1995. Difloxacin reverses multidrug resistance in HL-60/AR cells that overexpress the multidrug resistance-related protein (MRP) gene. Oncol. Res. 7:213-225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

